Clinical Features of Normal Pressure Hydrocephalus
Terry D. Fife, MD
Division of Neurology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Normal pressure hydrocephalus (NPH) is a condition characterized by a triad of progressive gait apraxia, cognitive deficits, and urinary incontinence in the context of relative ventricular enlargement and a cerebrospinal fluid (CSF) pressure less than 20 cm H2O. Gait disturbance is the most common presentation although symptoms may progress to psychomotor slowing, dementia, and the need for institutionalized care. NPH may result from subarachnoid hemorrhage, meningitis, trauma, or other causes, but it is often idiopathic. The mechanism is related to an insufficiency in CSF absorption that causes ventriculomegaly to develop gradually. Identification of patients with NPH is challenging because no single diagnostic study has emerged as highly reliable. The triad of symptoms combined with ventricular enlargement disproportionate to cortical atrophy continues to be the most common diagnostic standard used. CSF shunting may reverse the gait disturbance and, to a lesser extent, also may improve urinary and cognitive symptoms. Complications associated with CSF shunts remain a concern that underscores the importance of appropriate patient selection. This article discusses the clinical features of NPH that help identify patients most likely to benefit from treatment.
Key Words: cerebrospinal fluid shunts, chronic hydrocephalus, communicating hydrocephalus, dementia, gait apraxia, normal pressure hydrocephalus, programmable CSF shunts, urinary incontinence
In 1965 Hakim and Adams[1,27] described patients with shunt-responsive chronic hydrocephalus whose cerebrospinal fluid (CSF) pressure was in the normal range. Progressive ventricular enlargement in the presence of normal CSF pressure had previously been noted,[21,40] but the clinical features of the syndrome and its reversal by diverting CSF were important new concepts. The underlying mechanism of this condition, known as normal pressure hydrocephalus (NPH), is related to insufficient CSF absorption that may lead to symptoms from vascular effects, 60 elevated transmantal pressure,[12,29] axonal stretching of the periventricular white matter, and deformation of the brain parenchyma.[19,26] NPH may be idiopathic, or it may develop in the aftermath of cerebral hemorrhage, trauma, meningeal infections, or any conditions that reduce resorption of CSF through the arachnoid villae.
In the almost 40 years since the initial description of NPH, our ability to readily identify individuals for whom shunting will be effective remains limited.[55] The clinical features of NPH include the triad of dementia, gait ataxia, and urinary incontinence. Because the syndrome does not necessarily manifest with all three elements of the triad and because incontinence, memory loss, and imbalance are so common in older people, the clinical picture of NPH is not unique. The difficulty clarifying the diagnosis clinically and radiographically combined with the previously high rate of complications associated with shunting[9,28,31,42] has led many neurologists to hold a somewhat nihilistic view of NPH and its treatment. Furthermore, myths about NPH abound (Table 1).
The relatively recent development of programmable shunts should reduce associated complications and improve outcomes compared to traditional fixed-rate shunts.[63] The development of safer and more effective shunts should reinvigorate efforts to identify patients most likely to benefit from CSF shunting. This article focuses on the current clinical and diagnostic techniques useful in identifying patients with NPH suitable for treatment by CSF shunting.
Gait Abnormality
Gait impairment is the most common and often the first symptom in patients with NPH.[13,19,23,24,28,34,62] The spectrum of gait disturbance ranges from nonspecific imbalance to inability to walk and a magnetic retropulsive stance.[5] Initially, there may be slight tendency to veer with turns, slight slowing, and the appearance of mild instability.
Patients report unsteadiness but no vertigo or illusion of motion, lightheadedness, or weakness. It may be difficult for both physician and patient to distinguish the gait of early NPH from so-called senile gait or very early parkinsonism.[15] As the gait deteriorates, which may occur over months or years, the base of the gait widens and its speed and fluidity decline. Like the gait of patients with Parkinson’s disease, stride length shortens (marche a petit pas) and gait speed slows. There may be some degree of shuffling, decreased floor clearance,[16,18,33,48-50] and difficulty turning.[32] Unlike the gait associated with Parkinson’s disease, cogwheel rigidity, if present, is mild compared to the degree of gait difficulty. Other features that distinguish NPH from Parkinson’s disease are the absence of significant resting tremor or drooling and failure to improve when levodopa is administered. Cerebellar ataxia can be distinguished from NPH by the presence of dysarthria, gazeevoked nystagmus, and appendicular dysmetria, none of which are typical of chronic hydrocephalus. Gait apraxia may worsen to the point of “magnetic gait,” and patients appear to have forgotten how to take a step or even to stand.
Typically, gait ataxia is the most useful indication of NPH.[38] When gait disturbance is the predominant symptom of NPH, it implies an improved chance of responsiveness to CSF shunting.[19,20,23] Furthermore, gait abnormality is the most likely component of the symptom triad of NPH to improve with CSF diversion,[36,58] and the absence of gait ataxia predicts poor responsiveness to shunting.[4,25,30]
Although there is some correlation between ventricular size and gait apraxia,[19] the relationship is not entirely reliable. Some patients may exhibit severe gait impairment with only mild ventricular enlargement while others with very large ventricles walk surprisingly well.
Many conditions affect gait and a comprehensive evaluation is needed before concluding that gait difficulty is caused by NPH (Table 2).
Some patients with NPH reach medical attention because they exhibit prominent changes in mental status or because their gait difficulties are thought to be related to other known medical conditions. NPH probably causes fewer than 1% of all dementias[55] but is important to consider because some patients improve with CSF shunting.[53] Initially, there is slowing of mental processing, reduced organizational and problem-solving skills, and a reduction in prior interests. Patients may become more taciturn, less spontaneous in conversations, withdrawn, and apathetic. This abulia reflects bifrontal lobe dysfunction and leads to changes in personality, memory loss, and a reduced ability to perform mechanical activities or to think three dimensionally. [18] As the dementia progresses, patients may have difficulty finding words.
Aphasia, however, is not prominent in the dementia of NPH. By the time significant memory deficits appear, motor slowing and some degree of gait apraxia are typically evident. When the dementia is moderate to advanced, it may overlap clinically with Alzheimer’s dementia, Lewy body dementia, or subcortical dementias. A distinction can be made by the presence of a wide-based “magnetic” gait in NPH, which is not a feature of Alzheimer’s dementia.[59] Vascular dementia can be distinguished by magnetic resonance (MR) imaging of the brain showing scattered or extensive ischemic changes. Cognitive impairment is the least likely component of the clinical triad to recover.[34,43] If the dementia is severe, CSF shunting may result in little or no improvement.[34,37,44,53]
In a recent study, shunting of patients with probable Alzheimer’s dementia was not associated with a statistically significant improvement in dementia as assessed by a dementia rating scale and by the Mini-Mental Status Test after 1 year.[47] One reason for the poor response to shunting in patients with dementia may be that some have Alzheimer’s dementia or other degenerative disorders instead of or in addition to NPH.[22] Assessment of patients by the Mini-Mental Status Test or other neuropsychological tests can document cognitive deficits but adds little to the diagnosis or predictive outcomes associated with NPH.[45]
Urinary Incontinence
Disturbances of bladder control range from urinary frequency to incontinence. Incontinence typically signifies a late stage of NPH,[38] although some degree of urgency is often present.[19] Its prevalence is not known precisely. Some published studies have used urinary incontinence as an inclusion criterion, but it may be present in about half the patients diagnosed with NPH in published studies.[13] Bladder hyperactivity may improve temporarily with the removal of 50 ml of CSF, and it may improve or resolve with CSF shunting.[2,14,28]
Urinary frequency, urgency, and incontinence may result from a variety of common disorders, including prostatic hypertrophy, pelvic relaxation, cystitis, and other causes of a flaccid or spastic neurogenic bladder. The multitude of potential causes for urinary incontinence related to causes other than NPH may explain why its positive predictive value for improvement with shunting is only 33%.[3,4] Incontinence is not specific for NPH and appears to be a poor independent predictor of response to treatment.
Diagnosis
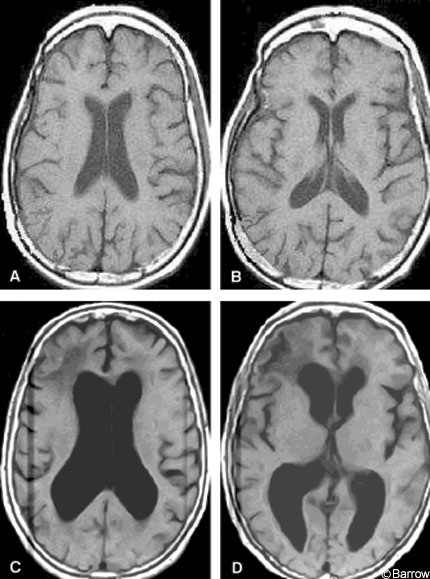
The diagnosis of NPH relies on the combination of historical features, examination, and findings on brain imaging. Slowly progressive gait apraxia over months to several years in the presence of ventricular enlargement should prompt consideration of NPH. Conditions to consider in the differential diagnosis of gait imbalance with a normal or nearly normal MR image are outlined in Table 2. All other possible causes of gait disturbance should be considered, including combinations of conditions, before narrowing the cause to NPH. Disequilibrium in older people may be caused by a variety of conditions.[17] Some individuals may have disequilibrium that responds to balance-training physical therapy so this option should be considered before proceeding to CSF shunting. Brain imaging is essential to arrive at the diagnosis of NPH. Although many tests and measurements have been proposed (Table 3) and some are useful, the most commonly used and practical measure is ventricular enlargement disproportionate to cortical atrophy on brain MR imaging or head computed tomography (CT, Fig.1).
 The absence of dementia or complaints of incontinence should not deflect consideration of NPH as a cause of gait apraxia.[20] Failure to respond to a CSF tap test does not necessarily indicate failure to respond to long-term shunting.[56]
The absence of dementia or complaints of incontinence should not deflect consideration of NPH as a cause of gait apraxia.[20] Failure to respond to a CSF tap test does not necessarily indicate failure to respond to long-term shunting.[56]
In most cases, the changes in the ventricles and central nervous system that cause NPH develop over months and years and may not always improve noticeably within the short timeframe used to assess responses to the tap test.[39]
Patients exhibiting a progressive decline in gait, ventricular enlargement disproportionate to the degree of atrophy, and in whom no other explanation of the gait disturbance is identified should be considered candidates for CSF shunting even if they lack substantial dementia or urinary incontinence.
Prognostic Indicators
A favorable response to a CSF tap test (removal of 30 to 50 ml CSF) or to 3 to 5 days of continuous lumbar drainage[11,13,61] suggests a likelihood of responding favorably to CSF shunting. A shorter duration of clinical symptoms (e.g., less than 6 months) and the onset of gait difficulty with little or no cognitive impairment[10,24] also suggest a better prognosis with CSF shunting. Early treatment may also be associated with greater improvement than treatment at later stages of NPH.[38]
Factors suggesting a lower likelihood of improvement include prior strokes or extensive white matter hypodensity on CT,[52] advanced or longstanding dementia, or dementia as the first neurological manifestation.[10,24,56] Long-standing symptoms and the development of personality changes or irritability and early frontal lobe dysfunction connote a worse prognosis.[51]
Features that do not have substantial predictive value for shunt responsiveness include the presence of atrophy, [46,54] results of Mini-Mental Status or neuropsychological tests,[45] absence of response to the removal of 30 to 50 ml of CSF,[56] lack of response to extended lumbar CSF drainage,[61] absence of the complete “triad” of symptoms,[20,38] concurrent periventricular lesions, or modest ischemic changes in the deep white matter.[35]
In an analysis of pooled published literature, Hebb et al.[28] found that 59% received a measurable benefit from shunting but over time significant improvement was sustained in only 29%. A large prospective study showed that 64% improved and 37% improved significantly. [6-8]
Treatment and Complications
The only widely accepted treatment of NPH is CSF shunting. The shunt most commonly placed is the ventriculoperitoneal shunt. Lumboperitoneal shunts and ventriculoatrial shunts also may be used in certain situations. There are a variety of shunt types, including differential pressure valves, flow-regulated valves, antisiphon devices and, more recently, programmable valves. Differential pressure valves open when the CSF pressure differential exceeds a certain pressure threshold in the upright position. Flow-regulated valves are designed to increase resistance to CSF flow with increasing pressure differentials to prevent excessively rapid drainage. Siphon-control devices are sometimes used together with a valve system to minimize the effects of orthostatic changes in CSF pressure. Programmable valves permit the noninvasive adjustment of the degree of CSF flow through the valve. Consequently, the degree of CSF shunting can be tailored to the patient without replacing the valve itself. Complications may result from catheter occlusion or infection or from overdrainage or underdrainage. Overdrainage can cause subdural hydromas or hematomas. With underdrainage, the hydrocephalus and related clinical features may fail to improve.
The complication rate of the pooled literature review was 38%; 22% needed subsequent shunt revision. Six percent died or had serious neurological deficits.[28] Other studies indicate a complication rate between 20 and 40%.[57] Complications include overdrainage that may cause low-pressure headaches and possibly subdural hygromas or hematomas as bridging veins tear from ventricular decompression. It is hoped that programmable shunts may avert the tendency for overshunting in many patients if the shunt is set at a higher opening pressure and gradually reduced in successive adjustments depending on the patient’s clinical status. Other complications include infection, intracerebral hemorrhage, seizure, shunt malfunction or occlusion, and failure to respond due to underdrainage. Programmable valves should reduce the complications associated with both overshunting and undershunting but has not yet been demonstrated unequivocally.[41,63]
Conclusions
NPH is a condition that remains difficult to diagnose but that may respond well to treatment. However, the treatment consists of placing a CSF shunt, and the procedure is associated with a significant complication rate, particularly in older patients. Making the diagnosis requires a thorough investigation to exclude other causes of gait ataxia. If the gait abnormality is consistent with the diagnosis, symptoms appear to be significant and progressive, and brain imaging confirms ventricular enlargement, the diagnosis should be considered. In some cases, the diagnosis of NPH will remain speculative. Such patients may be followed clinically until a decision can be made.
References
- Adams RD, Fisher CM, Hakim S, et al: Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure. A treatable syndrome. N Engl J Med 273:117-126, 1965
- Ahlberg J, Norlen L, Blomstrand C, et al: Outcome of shunt operation on urinary incontinence in normal pressure hydrocephalus predicted by lumbar puncture. J Neurol Neurosurg Psychiatry 51:105-108, 1988
- Benzel EC, Pelletier AL, Levy PG: Communicating hydrocephalus in adults: Prediction of outcome after ventricular shunting procedures. Neurosurgery 26:655-660, 1990
- Black PM: Idiopathic normal-pressure hydrocephalus. Results of shunting in 62 patients. J Neurosurg 52:371-377, 1980
- Blomsterwall E, Svantesson U, Carlsson U, et al: Postural disturbance in patients with normal pressure hydrocephalus. Acta Neurol Scand 102:284-291, 2000
- Boon AJ, Tans JT, Delwel EJ, et al: Dutch Normal-Pressure Hydrocephalus Study: Randomized comparison of low- and medium-pressure shunts. J Neurosurg 88:490-495, 1998
- Boon AJ, Tans JT, Delwel EJ, et al: Dutch Normal-Pressure Hydrocephalus Study: The role of cerebrovascular disease. J Neurosurg 90:221-226, 1999
- Boon AJ, Tans JT, Delwel EJ, et al: The Dutch Normal-Pressure Hydrocephalus Study. How to select patients for shunting? An analysis of four diagnostic criteria. Surg Neurol 53:201-207, 2000
- Bret P, Chazal J, Janny P, et al: Chronic hydrocephalus in adults [French]. Neurochirurgie 36:1-159, 1990
- Caruso R, Cervoni L, Vitale AM, et al: Idiopathic normal-pressure hydrocephalus in adults: Result of shunting correlated with clinical findings in 18 patients and review of the literature. Neurosurg Rev 20 :104-107, 1997
- Chen IH, Huang CI, Liu HC, et al: Effectiveness of shunting in patients with normal pressure hydrocephalus predicted by temporary, controlled-resistance, continuous lumbar drainage: A pilot study. J Neurol Neurosurg Psychiatry 57:1430-1432, 1994
- Conner ES, Foley L, Black PM: Experimental normal-pressure hydrocephalus is accompanied by increased transmantle pressure. J Neurosurg 61:322-327, 1984
- Damasceno BP, Carelli EF, Honorato DC, et al: The predictive value of cerebrospinal fluid taptest in normal pressure hydrocephalus. Arq Neuropsiquiatr 55:179-185, 1997
- Dixon GR, Friedman JA, Luetmer PH, et al: Use of cerebrospinal fluid flow rates measured by phase-contrast MR to predict outcome of ventriculoperitoneal shunting for idiopathic normalpressure hydrocephalus. Mayo Clin Proc 77:509-514, 2002
- Elble RJ, Hughes L, Higgins C: The syndrome of senile gait. J Neurol 239:71-75, 1992
- Estañol BV: Gait apraxia in communicating hydrocephalus. J Neurol Neurosurg Psychiatry 44:305-308, 1981
- Fife TD, Baloh RW: Disequilibrium of unknown cause in older people. Ann Neurol 34:694-702, 1993
- Fisher CM: The clinical picture in occult hydrocephalus. Clin Neurosurg 24:270-284, 1977
- Fisher CM: Hydrocephalus as a cause of disturbances of gait in the elderly. Neurology 32:1358-1363, 1982
- Fisher CM: Neuropathology in older people with disequilibrium of unknown cause. Neurology 54:1206-1207, 2000
- Foltz EL, Ward AA, Jr.: Communicating hydrocephalus from subarachnoid bleeding. J Neurosurg 13:546-566, 1956
- Golomb J, Wisoff J, Miller DC, et al: Alzheimer’s disease comorbidity in normal pressure hydrocephalus: Prevalence and shunt response. J Neurol Neurosurg Psychiatry 68:778-781, 2000
- Graff-Radford NR, Godersky JC: Normal-pressure
hydrocephalus. Onset of gait abnormality
before dementia predicts good surgical outcome. Arch Neurol 43:940-942, 1986 - Graff-Radford NR, Godersky JC, Jones MP: Variables predicting surgical outcome in symptomatic hydrocephalus in the elderly. Neurology 39:1601-1604, 1989
- Greenberg JO, Shenkin HA, Adam R: Idiopathic normal pressure hydrocephalus—a report of 73 patients. J Neurol Neurosurg Psychiatry 40:336-341, 1977
- Hakim CA, Hakim R, Hakim S: Normal-pressure hydrocephalus. Neurosurg Clin N Am 12:761-773, 2001
- Hakim S, Adams RD: The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 2:307-327, 1965
- Hebb AO, Cusimano MD: Idiopathic normal pressure hydrocephalus: A systematic review of diagnosis and outcome. Neurosurgery 49:1166-1184, 2001
- Hoff J, Barber R: Transcerebral mantle pressure in normal pressure hydrocephalus. Arch Neurol 31:101-105, 1974
- Hughes CP, Siegel BA, Coxe WS, et al: Adult idiopathic communicating hydrocephalus with and without shunting. J Neurol Neurosurg Psychiatry 41:961-971, 1978
- Illingworth RD, Logue V, Symon L, et al: The ventriculocaval shunt in the treatment of adult hydrocephalus. Results and complications in 101 patients. J Neurosurg 35:681-685, 1971
- Kaye JA, Grady CL, Haxby JV, et al: Plasticity in the aging brain. Reversibility of anatomic, metabolic, and cognitive deficits in normal-pressure hydrocephalus following shunt surgery. Arch Neurol 47:1336-1341, 1990
- Knutsson E, Lying-Tunnell U: Gait apraxia in normal-pressure hydrocephalus: Patterns of movement and muscle activation. Neurology 35:155-160, 1985
- Krauss JK, Regel JP, Vach W, et al: Vascular risk factors and arteriosclerotic disease in idiopathic normal-pressure hydrocephalus of the elderly. Stroke 27:24-29, 1996
- Krauss JK, Regel JP, Vach W, et al: White matter lesions in patients with idiopathic normal pressure hydrocephalus and in an age-matched control group: A comparative study. Neurosurgery 40:491-496, 1997
- Laws ER, Mokri B: Occult hydrocephalus: Results of shunting correlated with diagnostic tests. Clin Neurosurg 24:316-333, 1977
- Malm J, Kristensen B, Karlsson T, et al: The predictive value of cerebrospinal fluid dynamic tests in patients with the idiopathic adult hydrocephalus syndrome. Arch Neurol 52:783-789, 1995
- Meier U, Zeilinger FS, Kintzel D: Signs, symptoms and course of normal pressure hydrocephalus in comparison with cerebral atrophy. Acta Neurochir (Wien) 141:1039-1048, 1999
- Mesure S, Donnet A, Azulay JP, et al: Postural and locomotor evaluation of normal pressure hydrocephalus: A case report [French]. Rev Neurol (Paris) 157:1416-1419, 2001
- Penfield W: The principles of physiology involved in the management of increased intracranial pressure. Ann Surg 102:548-554, 1935
- Pollack IF, Albright AL, Adelson PD, et al: A randomized, controlled study of a programmable shunt valve versus a conventional valve for patients with hydrocephalus. Hakim-Medos Investigator Group. Neurosurgery 45:1399-1411, 1999
- Puca A, Anile C, Maira G, et al: Cerebrospinal fluid shunting for hydrocephalus in the adult: Factors related to shunt revision. Neurosurgery 29:822-826, 1991
- Raftopoulos C, Deleval J, Chaskis C, et al: Cognitive recovery in idiopathic normal pressure hydrocephalus: A prospective study. Neurosurgery 35:397-405, 1994
- Sand T, Bovim G, Grimse R, et al: Idiopathic normal pressure hydrocephalus: The CSF taptest may predict the clinical response to shunting. Acta Neurol Scand 89:311-316, 1994
- Savolainen S, Hurskainen H, Paljarvi L, et al: Five-year outcome of normal pressure hydrocephalus with or without a shunt: Predictive value of the clinical signs, neuropsychological evaluation and infusion test. Acta Neurochir (Wien) 144:515-523, 2002
- Schoonderwaldt HC, Cho Chia Yuen G, Colon EJ, et al: The preselection value of the Doppler LP test for shunt-therapy in patients with normal pressure hydrocephalus. J Neurol 225:15-24, 1981
- Silverberg GD, Levinthal E, Sullivan EV, et al: Assessment of low-flow CSF drainage as a treatment for AD: Results of a randomized pilot study. Neurology 59:1139-1145, 2002
- Stolze H, Kuhtz-Buschbeck JP, Drucke H, et al: Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J Neurol Neurosurg Psychiatry 70:289-297, 2001
- Sudarsky L, Simon S: Gait disorder in late-life hydrocephalus. Arch Neurol 44:263-267, 1987
- Sypert GW, Leffman H, Ojemann GA: Occult normal pressure hydrocephalus manifested by parkinsonism-dementia complex. Neurology 23:234-238, 1973
- Takeuchi T, Kasahara E, Iwasaki M: Clinical characteristics and indications for shunting in patients with idiopathic normal pressure hydrocephalus with brain atrophy (atypical idiopathic normal pressure hydrocephalus) [Japanese]. No Shinkei Geka 28:505-515, 2000
- Tans JT, Boon AJ: How to select patients with normal pressure hydrocephalus for shunting. Acta Neurochir Suppl 81:3-5, 2002
- Thomsen AM, Borgesen SE, Bruhn P, et al: Prognosis of dementia in normal-pressure hydrocephalus after a shunt operation. Ann Neurol 20:304-310, 1986
- Tsunoda A, Mitsuoka H, Bandai H, et al: Intracranial cerebrospinal fluid measurement studies in suspected idiopathic normal pressure hydrocephalus, secondary normal pressure hydrocephalus, and brain atrophy. J Neurol Neurosurg Psychiatry 73:552-555, 2002
- Vanneste JA: Three decades of normal pressure hydrocephalus: Are we wiser now? J Neurol Neurosurg Psychiatry 57:1021-1025, 1994
- Vanneste JA: Diagnosis and management of normal-pressure hydrocephalus. J Neurol 247:5-14, 2000
- Vanneste JA, Augustijn P, Dirven C, et al: Shunting normal-pressure hydrocephalus: Do the benefits outweigh the risks? A multicenter study and literature review. Neurology 42:54-59, 1992
- Vassilouthis J: The syndrome of normal-pressure hydrocephalus. J Neurosurg 61:501-509, 1984
- Verghese J, Lipton RB, Hall CB, et al: Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med 347:1761-1768, 2002
- Vorstrup S, Christensen J, Gjerris F, et al: Cerebral blood flow in patients with normal-pressure hydrocephalus before and after shunting. J Neurosurg 66:379-387, 1987
- Walchenbach R, Geiger E, Thomeer RT, et al: The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 72:503-506, 2002
- Wikkelsö C, Andersson H, Blomstrand C, et al: Normal pressure hydrocephalus. Predictive value of the cerebrospinal fluid tap-test. Acta Neurol Scand 73:566-573, 1986
- Zemack G, Romner B: Adjustable valves in normal-pressure hydrocephalus: A retrospective study of 218 patients. Neurosurgery 51:1392-1402, 2002