Finger Tapping and Brain Dysfunction: A Qualitative and Quantitative Study
George P. Prigatano, PhD
Beate Hoffmann†
Department of Clinical Neuropsychology, Barrow Neurological Institute, Mercy Healthcare Arizona, Phoenix, Arizona
†Department of Psychology, University of Hamburg, Hamburg, Germany
Abstract
Fifteen brain dysfunctional patients and 15 normal control subjects were videotaped while performing the Halstead Finger Tapping Test. Compared to control patients, finger tapping in brain dysfunctional patients was not only slower but showed a higher frequency of abnormal finger movements. Disturbances of motor inhibition, apraxia, or both may account for these qualitative findings.
Key Words : neuropsychology, rehabilitation, traumatic brain injury
In his search for measures of “biological intelligence,” Ward Halstead6 identified the Finger Oscillation (or Tapping) Test as one potentially useful measure. In this test, subjects are asked to place their index finger on a key while their hand rests comfortably on a board. Subjects are then instructed to tap as fast as possible for 10 seconds. The procedure is repeated until five trials have been obtained with each hand in which each score is within five taps of one another.
Provided the subject is “motivated” to tap as fast as possible11 and there are no peripheral injuries that might influence performance negatively, this “simple” task has proven quite useful. Crossvalidation studies have shown that the mean speed of finger tapping can distinguish brain dysfunctional patients from both medical control patients15 and psychiatric patients.12 Speed of finger tapping in the dominant and nondominant hands also relates to the severity of a traumatic brain injury (TBI). Dikmen et al.3 demonstrated that median speeds were related to the time it took TBI patients to respond to commands in a meaningful way.
Motor recovery after a mild to moderate TBI appears to be better for grip strength than for speed of finger tapping.5 This finding is compatible with the notion that “motor slowing” (in part measured by speed of finger tapping) is related to the severity of brain injury8 and therefore may be involved with the ability or lack of ability to recover. This latter notion is supported by a study that related speed of finger tapping to the achievement of rehabilitation goals after an acute cerebral vascular accident.13 Interestingly, it was the speed of finger tapping of the hand ipsilateral to the lesion (the so-called “unaffected hand”) that distinguished patients who achieved rehabilitation goals from those who failed to do so.
Although various studies have documented the usefulness of quantitative measures of finger tapping, to our knowledge no study has investigated qualitative features of finger-tapping performance in brain dysfunctional patients and normal control patients. This lack is curious because various brain lesions are known to affect the motor systems differentially.1,2 Moreover, clinical observation frequently reveals individual variations, not only in the number of taps/10 sec but in the tempo or consistency of the rate of tapping. Furthermore, some patients have difficulty inhibiting other finger movements while performing this task.
In this exploratory study, we therefore videotaped finger movements of patients performing the Halstead Finger Tapping Test and asked three questions: Do brain dysfunctional patients seen for clinical neuropsychological evaluation show qualitative features of finger movement not observed in normal individuals? If so, can the features be classified with specific patterns? And, finally, what is the frequency of such abnormal movements?
Methods and Materials
Subjects
Thirty-one patients consecutively referred to the Department of Clinical Neuropsychology at the Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center for neuropsychological evaluation served as the initial study sample. Each patient had a known or suspected brain disorder at the time of referral. Patients were evaluated from mid-October to mid-December 1996.
Patients were diagnosed as follows. First, 13 patients had moderate to severe TBIs as defined by an admitting Glasgow Coma Scale (GCS) score of 9 to 12 and 3 to 8, respectively. One patient had a clearly documented mild TBI (GCS = 13 to 15). Five patients met criteria for dementia of the Alzheimer’s type.9 One patient had cerebral anoxia and a resolving amnestic syndrome. Three patients had neoplasms of the brain (one astrocytoma, one oligodendroglioma, and one choroid plexus tumor). One patient had hydrocephalus with spina bifida. One patient met the DSM-IV criteria for dementia, but its etiology was undetermined. One patient met the DSM-IV criteria for moderate mental retardation. One patient met the DSM-IV criteria for specific learning disability. Four patients lacked an adequate medical history or neuropsychological assessment to permit a specific diagnosis.
From this larger sample of 31 patients, 15 patients (12 males, 3 females) were identified and age matched to 15 (6 males, 9 females) normal subjects who had no history of neurological disorder. These latter individuals consisted primarily of visiting students, friends, or family members of the two investigators. Four of the patients with a brain injury were left-handed and 11 were right-handed. All of the control subjects were right-handed. Table 1 compares the demographic characteristics of these two groups as well as the diagnosis of the patients eventually selected for data analysis.
Procedures
As a part of their clinical neuropsychological evaluation, each patient was administered the Halstead Finger Tapping Test.14 Signed informed consent was obtained for each individual. Subjects were seated comfortably at a table on which the finger tapper was placed. Subjects were shown the finger-tapping device and its use was explained. They were instructed to tap as fast as possible for 10 sec, using the index finger of their preferred or dominant hand first. Additionally, all subjects were explicitly told to try and keep their other fingers down, resting comfortably on the board when tapping. They were also asked to try and rest the heel of their hand on the board when performing the tapping task. The procedure was demonstrated by one of two investigators who tested all participants.
The standard procedure of the Halstead Finger Tapping Test was slightly altered. Instead of having the patient tap five consecutive trials with their preferred hand, they were given three trials with the preferred hand first, followed by three trials with the nonpreferred or nondominant hand. They were then given two to three more trials with each hand, depending on the range of their scores and signs of fatigue. The number of taps achieved in 10 sec for each trial and with each hand was obtained.
In addition to this quantitative measure, each patient and 11 of the 15 control subjects were videotaped while performing the task. The other four normal subjects were not videotaped, but the qualitative features of their finger tapping were observed and recorded by the examiners during the trials.
Data Analysis
The means and standard deviations of speed of finger tapping were calculated for both the right and left hands of the two groups. Standard t -tests were used to compare the differences between the groups. In addition, raw scores were converted to demographically corrected standard (T) scores (for age, education, gender, and handedness) using the norms from Heaton et al.7 This correction was necessary given that the control subjects differed from patients in terms of education and the ratio of males and females.
The videotapes of the patients and control subjects were reviewed in an attempt to describe qualitative features of the subjects’ finger movements during the finger-tapping task. Movements exhibited by the brain dysfunctional patients that were not observed in the control subjects were identified and recorded. The frequency of “abnormal” finger movements exhibited by the two groups during the finger-tapping task was compared by a x 2 analysis.
Results
Quantitative Findings
The speed of finger tapping was slower in the brain dysfunctional patients than in the control subjects in both the dominant and the nondominant hands when demographically corrected T-scores were compared (Table 2). The level of performance of the control subjects was within one standard deviation of the standard T-score mean of 50 (SD = 10) and suggests that their speed of performance, given age, education, gender, and handedness, was indeed average. Brain dysfunctional patients tended to have a mean T-score in the low 40s, suggesting a mild slowing of their speed of finger tapping, although greater variability was observed in patients compared to the control subjects. This finding is compatible with the fact that various brain dysfunctions influence neuropsychological performance differentially.

Qualitative Findings
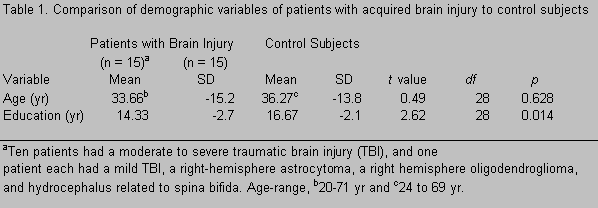
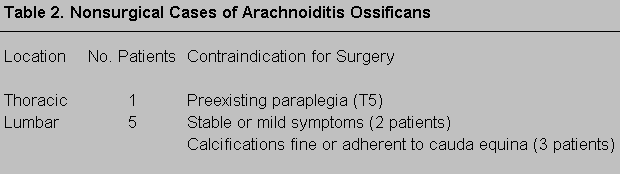
Four distinct patterns of finger movements were observed. Pattern 1 could be described as completely normal qualitative features of finger-tapping movements during the task. Subjects were able to tap their index finger while not moving or lifting fingers adjacent to the index finger while it was tapping (Fig. 1). In Pattern 1, the hand or adjacent fingers could move slightly while the index finger tapped, but the other fingers remained on the board as did the hand.
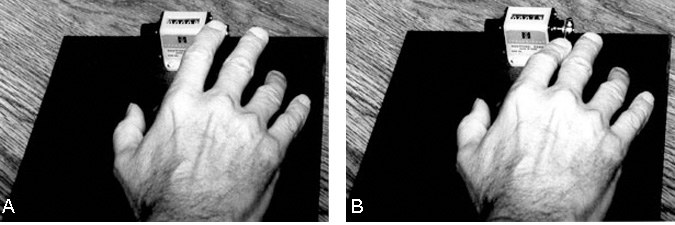
Pattern 2 was the first abnormal pattern observed (Fig. 2). It was difficult for the patient to inhibit movements of the middle finger while the index finger tapped. As the index finger depressed the lever, the adjacent finger lifted off the board and the patient apparently could not inhibit this movement despite repeated instructions to do so.
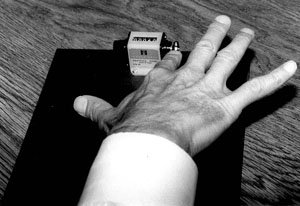
Pattern 3 was the second abnormal pattern observed (Fig. 3). Not only did the middle finger lift off the board while the index finger tapped, but the other fingers also failed to rest on the board. In some instances, the entire hand lifted off the board as the individual attempted to tap at fast rates of speed. This pattern was judged to occur when the subject could not inhibit the movement despite repeated instructions.
Pattern 4 , the third abnormal pattern, was observed in only one patient but its appearance was striking. This patient had an oligodendroglioma of the right hemisphere and tapped very slowly with the left hand (mean, 20 taps/10 sec). In contrast with the right hand, she was unable to coordinate finger movements and tended to show an exaggerated version of Pattern 3. This patient showed grossly dissimilar patterns in the two hands and thus Pattern 4 was considered a distinct type of movement.
Frequency of Patterns
Pattern 1 was observed in 14 of the 15 control subjects (i.e., 93.3%). The one exception was a 68-year-old female who exhibited Pattern 2. Pattern 1 was also observed in 7 of the 15 brain dysfunctional patients (46.6%). Altogether, eight patients (53.3%) with a brain injury exhibited a finger-tapping pattern with abnormal features. Pattern 2 was observed in three brain dysfunctional patients (one with a moderate and two with a severe TBI) (20%). Pattern 3 was observed in four brain dysfunctional patients (26.6%), all of whom had a severe TBI. Pattern 4 was observed in only the patient (6.6%) with the right hemisphere oligodendroglioma, as noted above. The frequency of “normal” versus “abnormal” finger movements in brain dysfunctional patients and controls was highly significant (x 2 = 7.78; df = 2; p = .005).
Discussion
This exploratory investigation has demonstrated two basic findings. First, it replicated the well-known finding that the mean speed of finger tapping in either the dominant or the nondominant hand may be considerably slower in brain dysfunctional patients than in normal control subjects. This finding reaffirms the usefulness of this quantitative measure.
In addition, more than 50% of brain dysfunctional patients exhibited abnormal qualitative features of finger movement seldom observed in young normal individuals. We have tentatively classified three such patterns of abnormal movements, which should be crossvalidated and studied in further detail.
Patients who exhibited Pattern 2 (i.e., who were unable to inhibit the middle finger) appeared to demonstrate a subtle form of motor disinhibition. That is, when requested, they seemed unable to inhibit the tendency of the adjacent finger to move while performing the task. The dorsal digital nerves derive from the medial branch of the radial nerve. The radial nerve innervates the thumb, index, middle, and ring fingers.10 These nerves bifurcate at the index and middle fingers and again at the middle and ring fingers. Perhaps central lesions compromise inhibitory control of this nerve at the point of bifurcation. When injuries are more severe, the inability to inhibit all fingers may be even greater, perhaps accounting for Pattern 3.
Although motor disinhibition may account for Patterns 2 and 3, it did not seem to account for Pattern 4. The single patient who showed this pattern demonstrated a mild left hemiparesis. The speed of finger tapping with the left hand was slow, but the form of the hand movement was normal. In contrast in the so-called “unaffected” hand on the right side, the patient showed notable difficulties coordinating finger movements. Clinically, this pattern appeared to be a form of apraxia. Geschwind4 has suggested that lesions anywhere in the brain may produce a disconnection syndrome that, in turn, can affect apraxia in one but not necessarily two hands. This mechanism might account for Pattern 4 as exhibited by the patient with the oligodendroglioma.
Further evaluation of the underlying neuropsychological disturbance associated with these abnormal finger movements may prove useful in the assessment and diagnosis of brain dysfunctional patients. For example, we have followed one patient with Alzheimer’s disease for several months (unpublished data). In the early stage of Alzheimer’s disease, her finger tapping movements were completely normal. In the middle stage, she began to show disinhibitory control of the fingers, particularly when encouraged to tap as fast as she could. Again, this so-called “simple” motor task may be quite useful for diagnosis and for tracking the course of a patient’s recovery.13
Conclusions
The speed of finger tapping in brain dysfunctional patients was not only slow, but the finger movements of more than 50% of these patients in this initial investigation showed abnormal qualitative features during the task. Further exploration of the underlying mechanisms responsible for slow and abnormal finger movements during the Halstead Finger Tapping Test may provide useful information for clinical neuropsychologists and behavioral neurologists.
References
- Angevine JB, Jr., Cotman CW: Principles of Neuroanatomy. New York: Oxford University Press, 1981
- Brooks VB: The Neural Basis of Motor Control. New York: Oxford University Press, 1986
- Dikmen S, Machamer JE, Winn HR, et al: Neuropsychological outcome at 1-year post head injury. Neuropsychology 9:80-90, 1995
- Geschwind N: The apraxias: Neural mechanisms of disorders of learned movement. Am Sci 63:188-195, 1975
- Haaland KY, Temkin N, Randahl G, et al: Recovery of simple motor skills after head injury. J Clin Exp Neuropsychol 16:448-456, 1994
- Halstead WC: Brain and Intelligence. Chicago: University of Chicago Press, 1947
- Heaton RK, Grant I, Matthews CG: Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Correlations, Research Findings, and Clinical Applications. Odessa, FL: Psychological Assessment Resources, 1991
- Klonoff PS, Costa LD, Snow WG: Predictors and indicators of quality of life in patients with closed-head injury. J Clin Exp Neuropsychol 8:469-485, 1986
- McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939-944, 1984
- Netter FH: Atlas of Human Anatomy. Summit, NJ: CIBA-Geigy, 1989
- Prigatano GP: Motivation and awareness in cognitive neurorehabilitation, in Stuss DT, Winocur G, Robertson IH (eds): Cognitive Neurorehabilitation: A Comprehensive Approach. New York: Cambridge (In press)
- Prigatano GP, Parsons OA: Relationship of age and education to Halstead test performance in different patient populations. J Consult Clin Psychol 44:527-533, 1976
- Prigatano GP, Wong JL: Speed of finger tapping and goal attainment after unilateral cerebral vascular accident. Arch Phys Med Rehabil 78:847-852, 1997
- Reitan RM: Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Indianapolis: Indiana University Medial Center, 1955
- Vega A, Jr., Parsons OA: Cross-validation of the Halstead-Reitan tests for brain damage. J Consult Clin Psychol 31:619-623, 1967