
Transcallosal Resection of Hypothalamic Hamartoma: Case Report
Yu-tze Ng, MD*/***
Harold L. Rekate, MD**
John F. Kerrigan, MD*/***
Erin Prenger, DO****
Jeffrey V. Rosenfeld, MD#
Robert F. Spetzler, MD**
Divisions of *Pediatric Neurology, **Neurological Surgery, ***Comprehensive Epilepsy Center, and ****Neuroradiology, Barrow Neurological Institute and Children’s Health Center, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
#Department of Neurosurgery, Alfred Hospital, Melbourne, Victoria, Australia
Abstract
Hypothalamic hamartomas are rare congenital malformations that typically become symptomatic with refractory seizures. Previously, neurosurgical resection of these tumors has been considered too difficult. When performed, surgery has been associated with significant residual tumor, complications, and nonresolution of the seizures. An 8-year-old boy underwent successful resection of a hypothalamic hamartoma through a transcallosal-interforniceal route. In the short-term postoperative period, the boy was seizure-free and his development and behavior improved.
Key Words: epilepsy surgery, gelastic seizures, hypothalamic hamartoma
Hypothalamic hamartomas are rare developmental malformations of the tuber cinereum.[6] They typically manifest with gelastic (laughing) seizures, which were first described by Daly and Mulder in 1957.[5] The gelastic seizures tend to be followed by the development of other types of seizures, including tonic, tonic-clonic, and complex partial.[9,25] Predominantly gelastic seizures, but also all seizure types, are extremely refractory to antiepileptic drugs (AEDs) and other medications. Some patients also experience precocious puberty, endocrinological abnormalities, and behavioral and cognitive decline (probably exacerbated by the seizures).
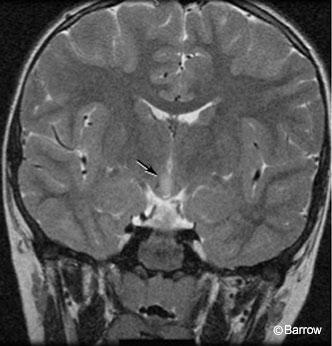
Arita and coworkers have classified hypothalamic hamartomas into two types on the basis of findings on magnetic resonance (MR) imaging.[1] The parahypothalamic type is attached to the floor of the third ventricle or a peduncle. This type is associated with precocious puberty. The intrahypothalamic type is enveloped by the hypothalamus and distorts the third ventricle. This type is more commonly associated with gelastic epilepsy, with or without precocious puberty, mental retardation, and behavioral problems. The sessile or intrahypothalamic types of hypothalamic hamartomas have a prominent intraventricular component. They are most strongly associated with gelastic epilepsy, probably because of their juxtaposition to the hypothalamus and central connections. [25] The clinical manifestations of a hypothalamic hamartoma also may depend on its size.[28]
Various therapies have had limited success in controlling the seizures: vagal nerve stimulation, corpus callosotomy, Gamma knife therapy (probably most successful), stereotactic radiofrequency destruction of the lesion, and even administration of a gonadotropin-releasing hormone analogue.[8,10,11,14,19,20,23,24,32] Rosenfeld et al. described excellent outcomes in several patients who underwent microsurgical resection of their hypothalamic hamartoma through a transcallosal-interforniceal route to the third ventricle using frameless stereotaxy.[25] We describe the successful resection of a hypothalamic hamartoma in an 8-year-old boy.
Illustrative Case
An 8-year-old boy began having gelastic seizures soon after birth. As is common, his condition was not diagnosed until he was about 2 years old. The patient had as many as 100 seizures a day, and complex partial and atonic seizures also developed. At consultation, his seizures had been reasonably well controlled by topiramate and tiagabine: He had only two or three gelastic seizures a day. He had failed therapy with seven other AEDs (lamotrigine, valproate, phenytoin, gabapentin, carbamazepine, clonazepam, and phenobarbital).
The patient had no signs of precocious puberty or any other endocrinological problems. He had a mild learning disability and was attending a special needs class in the first grade. He had tantrums and episodes of rage but had not required psychiatric intervention. His perinatal history was unremarkable, and his neurological examination was normal. MR imaging showed a 12×7 x10-mm mass in the hypothalamus with a hyperintense, T2-weighted signal slightly eccentric to the right (Fig. 1).
Surgical Technique
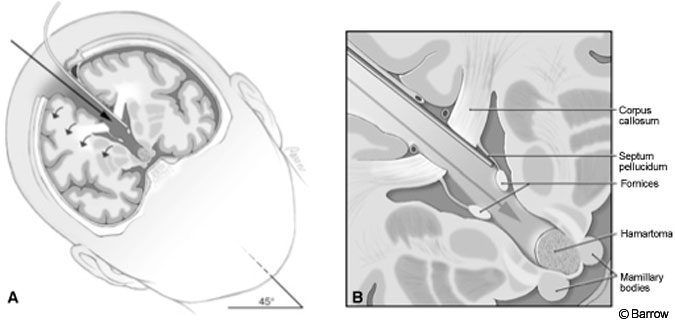
The procedure was performed with some modifications using the approach originally described by Rosenfeld and colleagues.[25] After induction and placement of arterial and central venous lines, the patient was positioned supine. His head was turned to the right, fixed in a Mayfield headholder (Codman, Inc., Raynham, MA), and elevated 30 degrees from the horizontal.
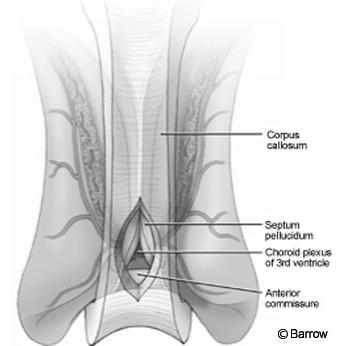
The craniotomy extended from just behind the coronal suture to a point 4 cm in front of the suture. Under frameless stereotactic guidance, the callosotomy was made in the midline about 3 cm long. Immediately below the corpus callosum in the midline, the triangular potential space was located (Fig.2). Identifying this space is the essential part of the procedure. The triangle is composed of the corpus callosum superiorly and by the two leaves of the septum pellucidum laterally. The apex of this triangle was explored gently with a Penfield No. 6 probe, which was used to separate the leaves of the septum pellucidum. As these leaves were separated, they spread the columns of the fornices without the need to place retractors on them (Fig. 3). A Greenberg retractor (Greenberg Retractor System, Codman, Inc., Raynham, MA) was placed on the leaf of the left septum pellucidum and falx. The right hemisphere and septum pellucidum were retracted by gravity. At this point the surgeon could view the floor of the third ventricle (i.e., the hypothalamus, Fig. 4).
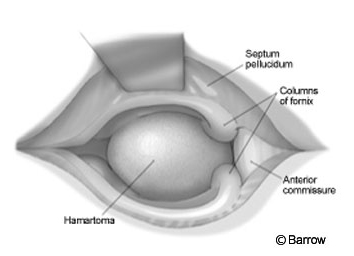
The hamartoma, found using frameless stereotactic images, was identified by a bulge distorting the ependymal surface of the hypothalamus. Typically, a hamartoma is light gray and easily distinguished from normal tissue. At the conclusion of the procedure the pial surface of the hypothalamus, the limit of the resection, was identified. Hemostasis was obtained, and the wound was closed in the standard fashion.
Postoperative Outcome
Immediately after surgery, the patient was able to move all four limbs well and to respond to his father’s commands. Soon thereafter he was extubated. Within 2 days he was walking and talking normally. Two doses of intravenous desmopressin acetate (10 µg and 2 µg) were administered although his serum sodium only dropped to 141 mEq/L. There was no evidence of diabetes insipidus.
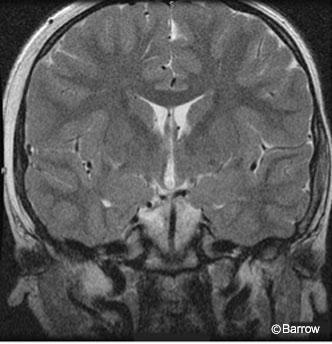
magnetic resonance image confirming
resection of the hypothalamic hamartoma.
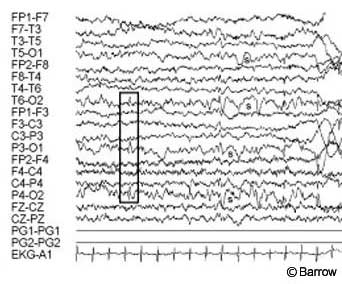
Postoperative MR imaging showed evidence of the frontal approach and no residual hypothalamic hamartoma (Fig. 5). Postoperative electroencephalography showed diffuse slowing with predominantly posterior spike activity only (Fig. 6). Pathological analysis was consistent with a hypothalamic hamartoma.
Seven weeks after surgery, the patient was seizure-free on his original two AEDs. His family reported that he was doing much better at school. They were extremely pleased with his progress.
Discussion
Resection of hypothalamic hamartomas has been considered too technically difficult and too unlikely to alleviate seizures to be worthwhile.[2] Partial and generalized (nongelastic) seizures have also been falsely localized to temporal and frontal regions, and those areas have been resected mistakenly as a consequence. Recently, increasing evidence has indicated that hypothalamic hamartomas are intrinsically epileptogenic and responsible for both pseudotemporal and generalized seizures. This notion is supported by the prolonged remission of seizures after surgical resection of these lesions. [3,9]
Multiple surgical approaches and techniques have been used to resect hypothalamic hamartomas: subfrontal, transsylvian, subtemporal, frontotemporal, pterional craniotomy, interhemispheric and translamina terminalis, and epidural subtemporopterygial approaches.[4,7,12,13,15-19,21,22,26,27,29-31] Unfortunately, many of these procedures are associated with high complication rates, including capsular and thalamic infarcts (with associated hemiparesis), third nerve palsy, and memory loss. More importantly, however, these procedures have often failed to provide adequate resection of the tumor, and seizure activity has persisted.
Rosenfeld and colleagues (personal communication, 2003) have now performed more than 30 successful hypothalamic hamartoma resections via the transcallosal-interforniceal route. They have successfully removed all or most of the tumor, and seizures have resolved in more than 60% of their patients. [9,25] Their most significant residual complication has been short-term memory loss, but this problem has occurred in very few patients.
In summary, hypothalamic hamartomas are rare but potentially devastating congenital malformations that cause the trademark gelastic seizures but also other types of seizures typically resistant to medical therapies. Severe cognitive, behavioral, and endocrinological complications are often associated with hypothalamic hamartomas. This case indicates that the transcallosal-intraforniceal route of resection is effective, safe, and, at present, the best form of treatment for hypothalamic hamartomas in appropriate patients.
Acknowledgment
We thank Maggie Varland, RN, BSB, Program Coordinator for the Hypothalamic Hamartoma Program, who was tireless in her efforts to coordinate the patient, surgery, and physicians.
References
- Arita K, Ikawa F, Kurisu K, et al: The relationship between magnetic resonance imaging findings and clinical manifestations of hypothalamic hamartoma. J Neurosurg 91:212-220, 1999
- Berkovic SF, Andermann F, Melanson D, et al: Hypothalamic hamartomas and ictal laughter: Evolution of a characteristic epileptic syndrome and diagnostic value of magnetic resonance imaging. Ann Neurol 23:429-439, 1988
- Berkovic SF, Kuzniecky R, Andermann F: Human epileptogenesis and hypothalamic hamartomas: New lessons from an experiment of nature. Epilepsia 38:1-3, 1997
- Breningstall GN: Gelastic seizures, precocious puberty, and hypothalamic hamartoma. Neurology 35:1180-1183, 1985
- Daly DD, Mulder DW: Gelastic epilepsy. Neurology 7:189-192, 1957
- Diebler C, Ponsot G: Hamartomas of the tuber cinereum. Neuroradiology 25:93-101, 1983
- Doshi PK, Polkey CE, Bullock P: Neurosurgical treatment of hypothalamic hamartoma causing gelastic epilepsy (abstract). J Neurol Neurosurg Psychiatry 59:658, 1995
- Dunoyer C, Ragheb J, Resnick T, et al: The use of stereotactic radiosurgery to treat intractable
childhood partial epilepsy. Epilepsia 43:292-300, 2002 - Freeman JL, Harvey AS, Rosenfeld JV, et al: Generalized epilepsy in hypothalamic hamartoma: Evolution and postoperative resolution. Neurology 60:762-767, 2003
- Fukuda M, Kameyama S, Wachi M, et al: Stereotaxy for hypothalamic hamartoma with intractable gelastic seizures: Technical case report. Neurosurgery 44:1347-1350, 1999
- Kuzniecky R, Guthrie B, Mountz J, et al: Intrinsic epileptogenesis of hypothalamic hamartomas in gelastic epilepsy. Ann Neurol 42:60-67, 1997
- Machado HR, Hoffman HJ, Hwang PA: Gelastic seizures treated by resection of a hypothalamic hamartoma. Childs Nerv Syst 7:462-465, 1991
- Matustik MC, Eisenberg HM, Meyer WJ 3rd: Gelastic (laughing) seizures and precocious puberty. Am J Dis Child 135:837-838, 1981
- Murphy JV, Wheless JW, Schmoll CM: Left vagal nerve stimulation in six patients with hypothalamic hamartomas. Pediatr Neurol 23:167-168, 2000
- Nishio S, Fujiwara S, Aiko Y, et al: Hypothalamic hamartoma. Report of two cases. J Neurosurg 70:640-645, 1989
- Nishio S, Morioka T, Fukui M, et al: Surgical treatment of intractable seizures due to hypothalamic hamartoma. Epilepsia 35:514-519, 1994
- Northfield DW, Russell DS: Pubertas praecox due to hypothalamic hamartoma: Report of two cases surviving surgical removal of the tumour. J Neurol Neurosurg Psychiatry 30:166-173, 1967
- Paillas JE, Roger J, Toga M, et al: Hamartoma of the hypothalamus. Clinical, radiological and histological study. Results of excision [French]. Rev Neurol (Paris) 120:177-194, 1969
- Palmini A, Chandler C, Andermann F, et al: Resection of the lesion in patients with hypothalamic hamartomas and catastrophic epilepsy. Neurology 58:1338-1347, 2002
- Parrent AG: Stereotactic radiofrequency ablation for the treatment of gelastic seizures associated with hypothalamic hamartoma. Case report. J Neurosurg 91:881-884, 1999
- Pendl G: Gelastic epilepsy in tumors of the hypothalamic region, in Penzholz H, Brock M, Hamer J, et al (eds): Advances in Neurosurgery 3. Brain Hypoxia. Pain. New York: Springer-Verlag, 1975, pp 442-447
- Polkey C, Chandler C, Doshi PK, et al: Resection of hypothalamic hamartoma for intractable epilepsy (abstract). Epilepsia 38 (Suppl 8):77, 1997
- Regis J, Bartolomei F, de Toffol B, et al: Gamma knife surgery for epilepsy related to hypothalamic hamartomas. Neurosurgery 47:1343-1352, 2000
- Regis J, Bartolomei F, Hayashi M, et al: The role of gamma knife surgery in the treatment of severe epilepsies. Epileptic Disord 2:113-122, 2000
- Rosenfeld JV, Harvey AS, Wrennall J, et al: Transcallosal resection of hypothalamic hamartomas, with control of seizures, in children with gelastic epilepsy. Neurosurgery 48:108-118, 2001
- Sato M, Ushio Y, Arita N, et al: Hypothalamic hamartoma: Report of two cases. Neurosurgery 16:198-206, 1985
- Sher PK, Brown SB: Gelastic epilepsy. Onset in neonatal period. Am J Dis Child 130:1126-1131, 1976
- Sturm JW, Andermann F, Berkovic SF: “Pressure to laugh”: An unusual epileptic symptom associated with small hypothalamic hamartomas. Neurology 54:971-973, 2000
- Takeuchi J, Handa H, Miki Y, et al: Precocious puberty due to a hypothalamic hamartoma. Surg Neurol 11:456-460, 1979
- Valdueza JM, Cristante L, Dammann O, et al: Hypothalamic hamartomas: With special reference to gelastic epilepsy and surgery. Neurosurgery 34:949-958, 1994
- Watanabe T, Enomoto T, Uemura K, et al: Gelastic seizures treated by partial resection of a hypothalamic hamartoma. No Shinkei Geka 26:923-928, 1998
- Zaatreh M, Tennison M, Greenwood RS: Successful treatment of hypothalamic seizures and precocious puberty with GnRH analogue. Neurology 55:1908-1910, 2000
