
High-Resolution Magnetic Resonance Imaging of Hypothalamic Hamartomas
Erin Prenger, DO
Joseph Heiserman, MD, PhD
David Amstutz, MD
Division of Neuroradiology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Hypothalamic hamartomas, rare lesions of the floor of the third ventricle, manifest with a spectrum of clinical symptoms that includes precocious puberty, gelastic seizures, and behavioral disorders. Recent advances in high-resolution magnetic resonance (MR) imaging and functional MR imaging have improved our ability to detect and classify these lesions. Based on glial-to-neuronal cell ratios, preliminary data indicate that there may be unique histological subtypes of hamartomas.
Key Words: hamartoma, hypothalamus, MR imaging, MR spectroscopy
Hamartomas, a mixture of indigenous cell lines arranged in a disorganized fashion, can occur anatomically in various locations throughout the central nervous system. They may exert tumor-like mass effect on adjacent tissue, but they lack the microscopic features of cellular proliferation. Hamartomas are often clinically silent because they are small and their natural history is benign. However, lesions that arise in the region of the floor of the third ventricle, specifically near the tuber cinereum and hypothalamus, manifest with a unique spectrum of symptoms, including precocious puberty, seizures, and behavioral disorders.[1,2] Their variation in size at presentation, histological similarity to surrounding tissue, and location centrally within the complex structures of the floor of the third ventricle present unique challenges for imaging these lesions. The relatively recent advent of high-resolution magnetic resonance (MR) imaging and functional MR imaging such as MR spectroscopy has helped improve the detection and delineation of hypothalamic hamartomas.
Anatomic Considerations
Hamartomas of the third ventricle arise from the region of the tuber cinereum or from the hypothalamus along the base of the lateral walls of the third ventricle. The floor of the third ventricle originates anteriorly at the lamina terminalis and extends posteriorly to the level of the aqueduct of Sylvius. When viewed in the coronal plane, the ventricular floor appears as a thin membrane bridging the inferior aspects of the hypothalamus, separating the third ventricle superiorly from the suprasellar cistern inferiorly. When viewed in the sagittal plane, the floor of the third ventricle begins anteriorly at the inferior margin of the lamina terminalis, extends slightly inferiorly to form the optic recess, drapes above the optic chiasm, and then extends inferiorly again to form the infundibular recess. Immediately posterior to the infundibular recess a small, funnel-shaped depression extends inferiorly from the floor of the third ventricle, giving rise to the pituitary infundibulum. This hollow eminence of gray matter extending from the optic chiasm to the mamillary bodies is the tuber cinereum. It is limited posteriorly by the mamillary bodies and laterally by the inferomedial nuclei of the hypothalamus. Hamartomas that arise in the region of the tuber cinereum can extend anteriorly to the infundibulum and optic chiasm, posteriorly to the mamillary bodies and basilar artery, inferiorly into the suprasellar and prepontine cistern, and superolaterally into the hypothalamus and third ventricle.
Anatomically, hypothalamic hamartomas can be divided into two broad categories: sessile and pedunculated.[1] Pedunculated lesions extend in an exophytic fashion inferiorly from the tuber cinereum and involve the infundibulum to various degrees. Patients with pedunculated lesions typically become symptomatic with precocious puberty. Sessile lesions arise from the inferomedial hypothalamus, near the junction of the lateral wall of the third ventricle and the tuber cinereum. Sessile hamartomas tend to extend medially, superiorly, and inferiorly to fill the inferior third ventricle and superior aspect of the suprasellar cistern. Patients with sessile hypothalamic hamartomas can manifest with seizures, typically gelastic, although they also can exhibit various degrees of hypothalamic-pituitary dysfunction. The anatomic locations of these lesions overlap just as their clinical presentations overlap. Predominantly sessile lesions in patients with gelastic seizures can have a large pedunculated component extending into the suprasellar cistern. Alternatively, pedunculated lesions extending along the infundibulum in patients with precocious puberty can extend superiorly into the hypothalamus, along the lateral wall of the third ventricle. Patients with pedunculated lesions who are symptomatic only with precocious puberty are now treated successfully pharmacologically. Patients with sessile lesions and refractory seizures are candidates for surgical treatment. This discussion focuses on the latter.
MR Imaging
In patients with refractory seizures, hypothalamic hamartomas can be tiny, 1- to 2-mm lesions embedded in the inferomedial wall of the hypothalamus. As when imaging all patients with seizures, it is critical that patients suspected of harboring a hypothalamic hamartoma be imaged with sufficient resolution to detect extremely small lesions with imaging characteristics almost identical to those of adjacent tissue within the complex structures at the base of the brain.
The signal characteristics of hypothalamic hamartomas are typically similar to those of the adjacent hypothalamus and cortical gray matter: intermediate signal intensity on both T1- and T2- weighted images without enhancement after contrast administration. However, some lesions show increased signal intensity on intermediate- and T2-weighted sequences relative to gray matter. Hypothalamic hamartomas are therefore detected primarily due to their architectural disturbance within the region as opposed to being conspicuous due to a signal abnormality on MR imaging. High accuracy is associated with high resolution, thin-slice contiguous imaging. High-field imaging with at least 1.5 Tesla field strength is mandatory. At our institution, we use a 3 Tesla imaging system for most of these studies. This field strength doubles the signal-to-noise ratio compared to the more common 1.5 Tesla systems and thus allows higher in-plane resolution and thinner sections.
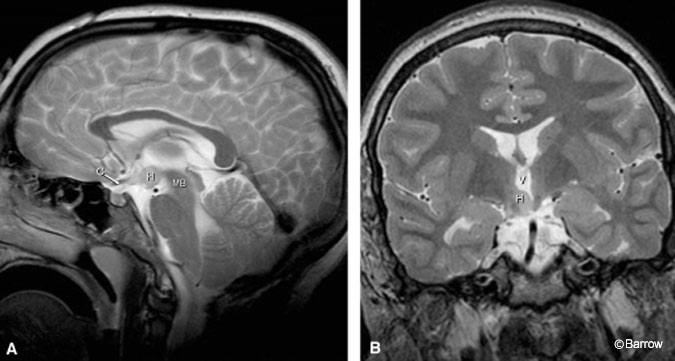
Small sessile hamartomas are best appreciated on coronal T2-weighted images, arising from the inferior and lateral walls of the third ventricle and extending to and involving the floor of the third ventricle (Fig. 1). On sagittal images they appear as an additional small nodule or gray matter anterior to the mamillary bodies and posterior to the infundibulum along the floor of the third ventricle.
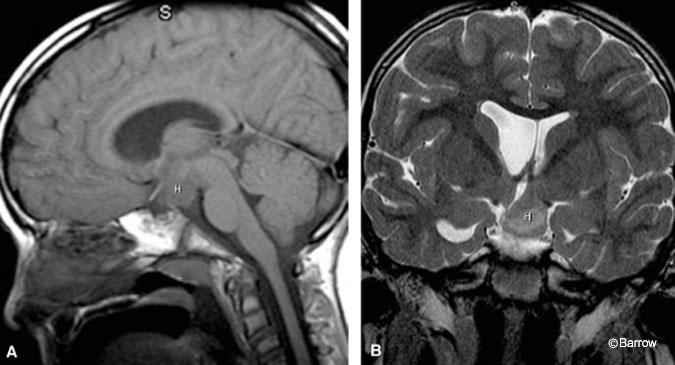
In patients with large hamartomas, it is important to delineate precisely the extent and origin of the lesion for preoperative planning because this information influences patient positioning and side of surgical approach. On coronal images, large sessile hamartomas appear to fill the entire third ventricle and contact both lateral walls of the third ventricle. Typically, however, these lesions arise from the base of one lateral wall of the hypothalamus and extend across the midline to contact the contralateral wall of the third ventricle (Fig. 2). The margin of the hamartoma contralateral to the side of its origin is therefore covered with ependyma apposed to the ependyma of the contralateral third ventricular wall. The ipsilateral margin of the lesion is contiguous with the hypothalamus. The extent of involvement with the hypothalamus itself is best delineated on coronal T2- weighted images, which allow visualization of the optic apparatus and fornices. Large lesions can extend anteriorly to the optic chiasm and posteriorly to the basilar artery but, in our experience, do not encase and only rarely displace these structures. Inferiorly extending lesions can fill the suprasellar cistern and prepontine cistern, exerting mass effect on the ventral pons. Large hamartomas can extend superiorly to fill the third ventricle to the level of the foramen of Monro. The internal architecture of large hamartomas can vary, exhibiting areas of signal hyperintensity and hypointensity on T2-weighted images. Frank necrosis and cyst formation have not been seen and, if present, would suggest an alternative diagnosis.
MR Spectroscopy
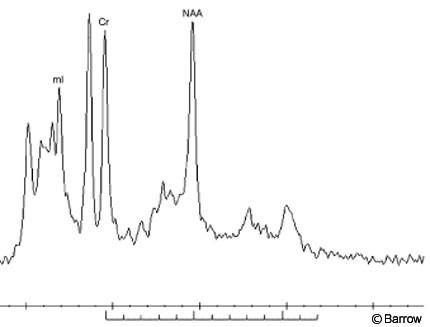
In addition to the superb soft-tissue contrast and high resolution offered by conventional MR imaging sequences, MR techniques also can be used to produce molecular spectra of a selected volume of interest. In particular, proton MR spectroscopy can be used to provide a new window on the cellular composition of lesions such as hypothalamic hamartomas. This technique can be performed in a single (2-4 cc) voxel or in a multivoxel mode allowing individual spectra to be obtained from a number of volumes of interest. Again, the increased signal available on the recently released 3 Tesla imaging systems is advantageous and allows smaller voxels to be sampled. In general, point resolved spectra (PRESS) methods with short echo times are used for maximum signal and peak identification. Because hypothalamic hamartomas lie near the base of the brain and adjacent to bony structures, voxel placement and accurate shimming are essential to successful spectroscopy. Individual peaks in the spectrum identify the presence of complex biomolecules associated with various cellular components (Fig. 3). For example, N-acetyl aspartate, one of the principal peaks seen in MR spectroscopy of the brain, arises primarily from neurons. Similarly, choline and myoinositol are associated with glial components. Little has been published on the spectroscopy of hypothalamic hamartomas;[3,4] however, preliminary work suggests that a distinctive MR spectroscopy signature is associated with these lesions. This signature allows hamartomas to be differentiated noninvasively from other possibilities, such as hypothalamic gliomas and metastatic deposits, before surgery. Ongoing work at our institution suggests that subtle spectral differences may identify subclasses of hamartomas based on histologic differences such as the glial cell-to-neuron ratio.
Conclusion
Recent advances in MR imaging have opened the window to the world of neuroanatomy and neuropathology. Anatomic resolution has improved dramatically. Tiny lesions that previously went undetected are now clearly visible. With recent advances in functional MR imaging, pathologic diagnosis is now aided by analysis of cellular composition based on the chemical fingerprint of MR spectroscopy. These new imaging tools continue to expand our understanding of unique neuropathological entities such as hypothalamic hamartomas.
References
- Arita K, Ikawa F, Kurisu K, et al: The relationship between magnetic resonance imaging findings and clinical manifestations of hypothalamic hamartoma. J Neurosurg 91:212-220, 1999
- Sturm JW, Andermann F, Berkovic SF: “Pressure to laugh”: An unusual epileptic symptom associated with small hypothalamic hamartomas. Neurology 54:971-973, 2000
- Tasch E, Cendes F, Li LM, et al: Hypothalamic hamartomas and gelastic epilepsy: A spectroscopic study. Neurology 51:1046-1050, 1998
- Wakai S, Nikaido K, Nihira H, et al: Gelastic seizure with hypothalamic hamartoma: Proton magnetic resonance spectrometry and ictal electroencephalographic findings in a 4-yearold girl. J Child Neurol 17:44-46, 2002
