Glioblastoma
Glioblastoma Overview
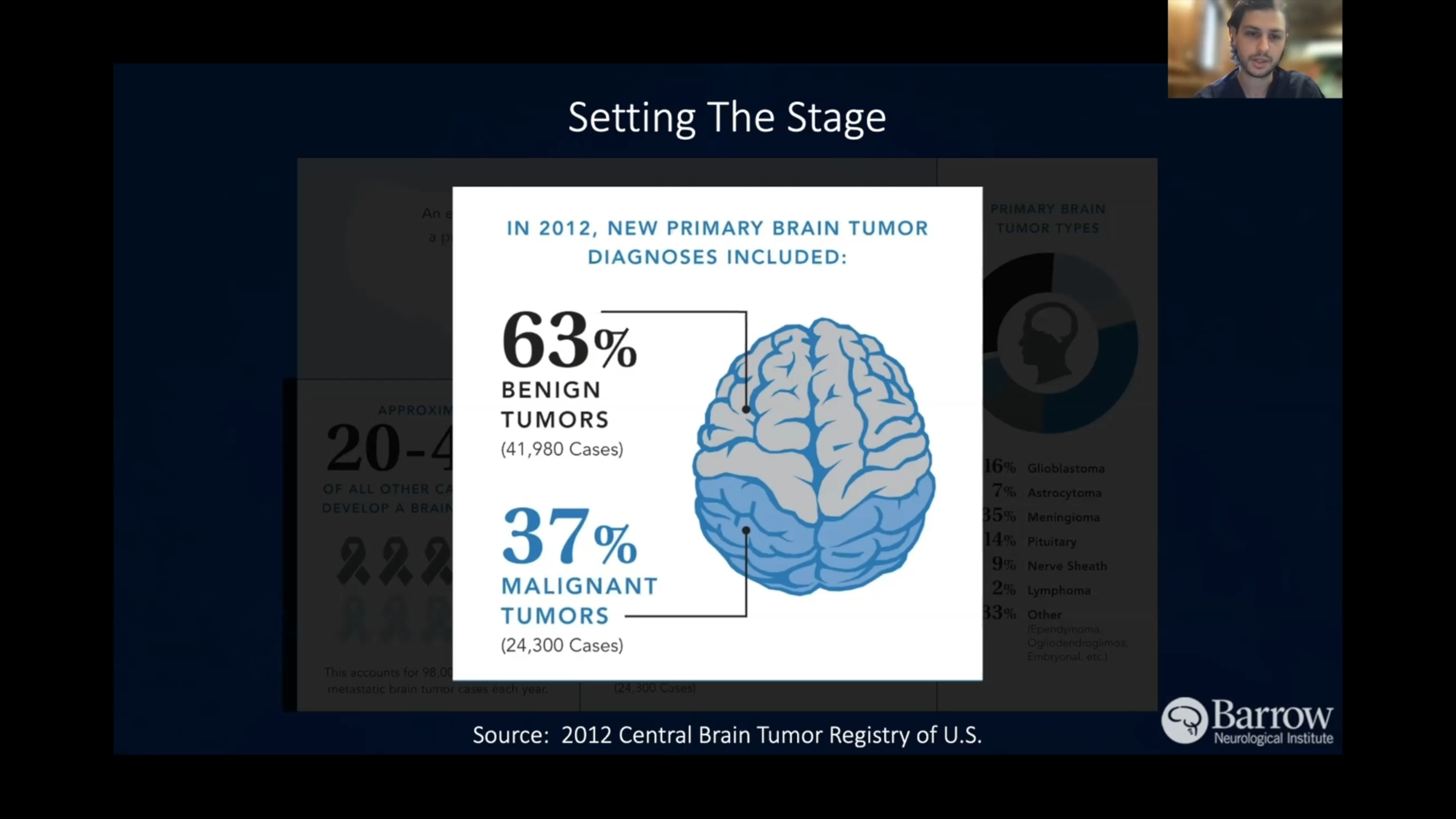
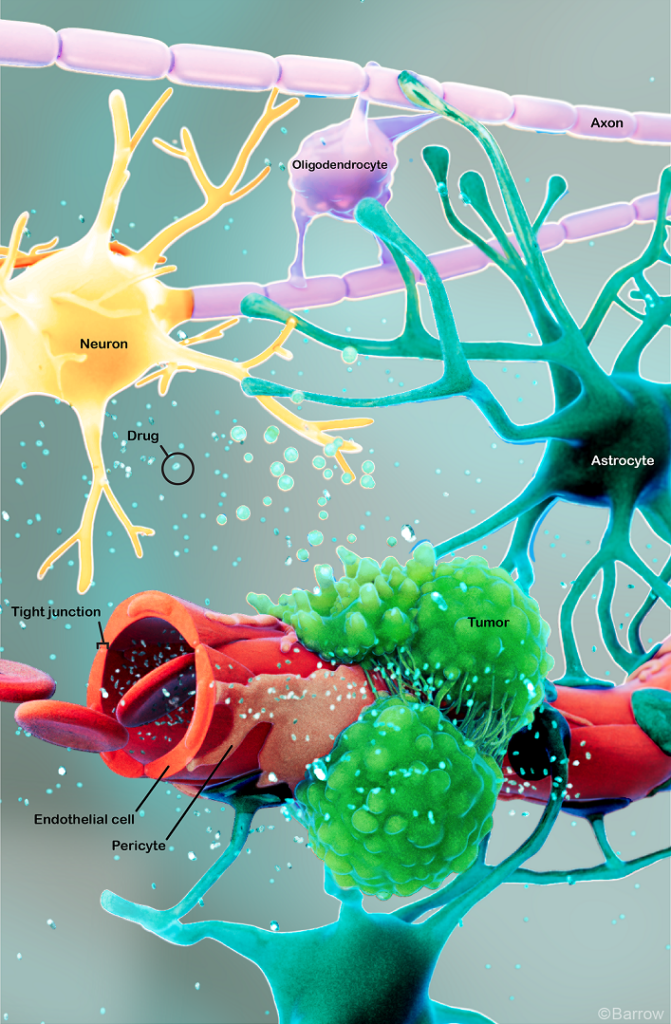
Glioblastoma tumors—formerly called glioblastoma multiforme (GBM) and occasionally called gliomas or high-grade astrocytomas—are malignant or cancerous brain tumors. Scientists are attempting to discover what kind of cells within the brain and spinal cord give rise to glioblastomas, with possibilities including neural stem cells or cells called astrocytes. Astrocytes are star-shaped cells that provide the brain and spinal cord with nutrients and help protect them from diseases in the blood.
Although glioblastomas rarely spread to other parts of the body, they’re quick to invade other parts of the brain. The World Health Organization (WHO) defines all glioblastomas as grade 4 brain tumors, meaning they’re the most aggressive type.
Glioblastoma is most commonly found in the brain’s frontal lobe, followed by the temporal, parietal, and occipital lobes. Spinal cord glioblastomas account for less than 5% of all glioblastomas.
What causes a glioblastoma?
As with most brain tumors, the exact cause of glioblastomas is not fully understood. Genetic, environmental, and lifestyle factors likely play a role, but more research is needed to pinpoint their specific mechanisms.
Risk factors identified in glioblastoma development include:
- Age: Glioblastomas are more common in older adults, with the risk increasing with age.
- Gender: Glioblastomas are slightly more common in males than females.
- Genetic mutations: Changes in specific genes, such as EGFR, TERT, and TP53 gene mutations, and specific chromosomal abnormalities have been linked to glioblastoma development. These mutations can disrupt normal cell growth and division, leading to the uncontrolled growth of cancer cells.
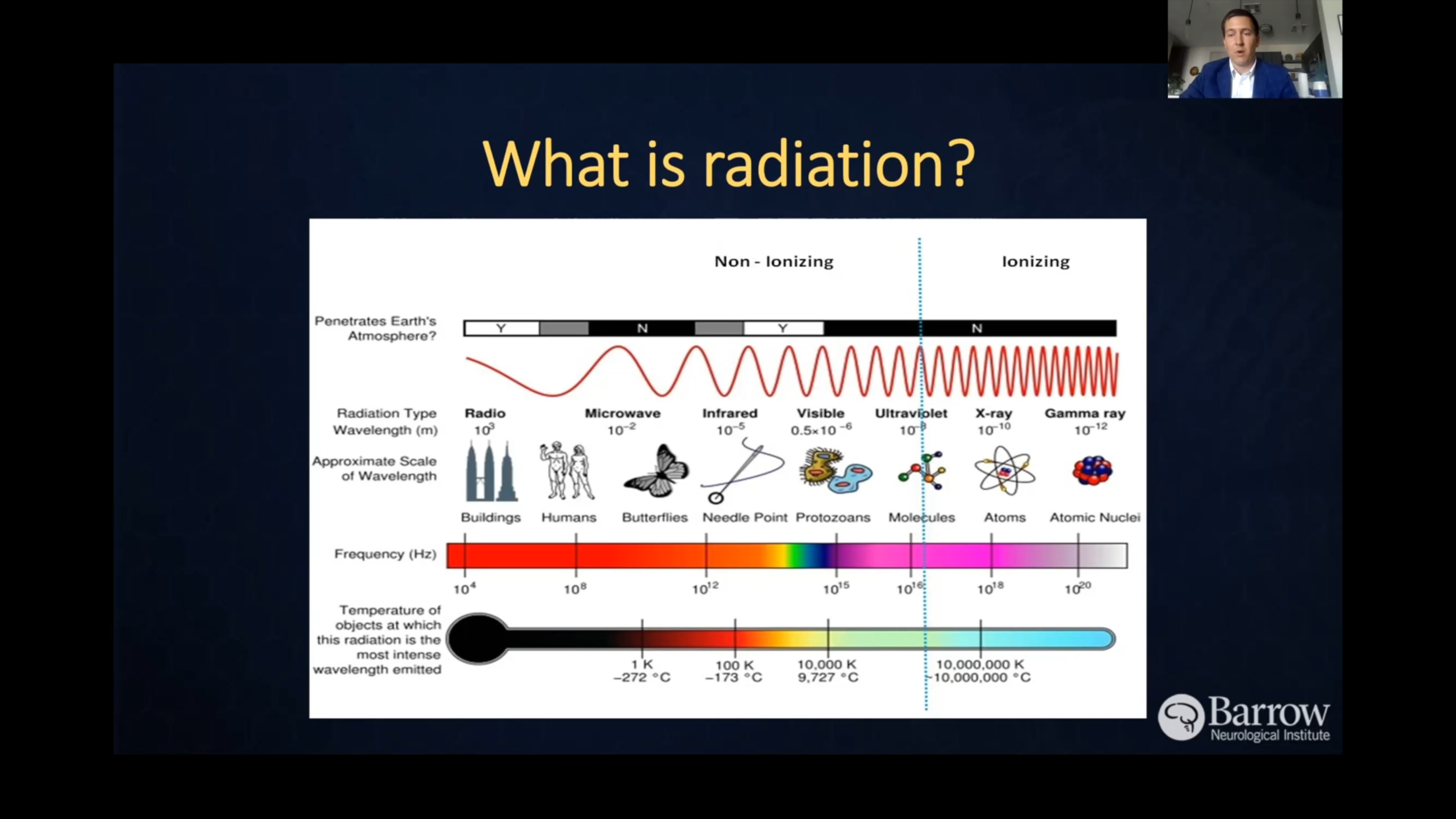
- Radiation exposure: Previous exposure to ionizing radiation—like radiation therapy for other cancers—is a known risk factor for glioblastoma. However, this accounts for only a small percentage of cases.
- Environmental factors: Although there isn’t a conclusive link, exposure to certain chemicals, like pesticides, solvents, and industrial pollutants, has been investigated as a potential risk factor for glioblastoma.

Glioblastoma Symptoms
Symptoms vary widely depending on a glioblastoma’s type, location, and size. Glioblastoma symptoms usually develop rapidly and are caused by the tumor itself or by the fluid surrounding the tumor causing additional brain swelling.
Symptoms you might notice if you or someone you know has a brain tumor can include:
- Headache: Headaches are often the first symptom of glioblastoma, including headaches that don’t improve with typical remedies, grow in frequency and intensity, or worsen with activity.
- Nausea and vomiting: Increased pressure inside the skull from the tumor’s aggressive nature can result in nausea and vomiting.
- Seizures: A brain tumor can cause seizures because it disrupts the brain’s regular electrical activity. Seizures can range from muscle jerks and spasms to a loss of consciousness and body functions. They can also resemble a vision, smell, or sensation change without losing consciousness.
- Fatigue and drowsiness: Glioblastomas interfere with normal brain functions, including those regulating sleep and wake cycles. They can also cause an increase in the pressure in the skull, which disrupts normal brain tissue and leads to symptoms like fatigue, drowsiness, and lethargy.
- Weakness on one side of the body: A tumor in the brain’s frontal lobe or brainstem may cause weakness in one limb or one side of the body, not both.
- Mental acuity: Glioblastomas can cause changes in mental clarity, alertness, and consciousness, ranging from mild confusion to coma in severe cases.
- Speech difficulties: This can look like a change in speech, hearing, or memory, like problems understanding or retrieving words.
- Vision changes: Vision changes, like partial vision loss or even double vision, can result from a tumor in the temporal lobe, occipital lobe, or brain stem.
- Personality or behavior changes: These can manifest as new or unusual emotional states, such as changes in judgment, aggressiveness, loss of initiative, and even sluggishness. Depression and anxiety, mainly if either develops suddenly, can be an early symptom of a tumor in the brain.
- Sensory changes: Changes in the ability to hear, smell, or see can be a symptom of a glioblastoma, and individuals may lose some of their ability to feel heat, cold, and pressure.
It’s important to note that these symptoms can overlap with those caused by other conditions. However, if you or someone you know is experiencing any of these symptoms—especially if they’re persistent or severe—it’s critical to consult a healthcare professional for evaluation. Early detection often improves outcomes.
Glioblastoma Diagnosis
A glioblastoma diagnosis generally requires the following steps to accurately diagnose the type, location, and extent of the tumor. The following exams and imaging options are frequently used for diagnosis:
- Physical and neurological exam: First, your healthcare provider will ask about your symptoms, overall health, and any risk factors you might have for a glioblastoma. Next, they’ll complete a neurological examination to assess neurological function, including reflexes, coordination, strength, and sensation.
- Magnetic Resonance Imaging (MRI): An MRI is the primary imaging test used to diagnose a glioblastoma. It provides detailed images of the brain and helps identify the tumor’s location, size, and characteristics.
- Computed Tomography (CT): Although an MRI is often the first choice in neuroimaging, a CT scan can help evaluate tumor size and location by relying on X-rays to create detailed cross-sectional brain images.
- Biopsy: In most cases, a definitive diagnosis of glioblastoma requires a biopsy. A biopsy establishes an exact diagnosis by removing a small tissue sample from the tumor surgically or through stereotactic biopsy. Neuropathologists then examine the tissue under a microscope to determine the type of cells present, whether they’re cancerous or non-cancerous, and other important characteristics that guide treatment decisions, like grade and genetic mutations. Though a glioblastoma may be suspected based on imaging studies alone, a tissue biopsy is needed to determine tumor type accurately.
Your care team may conduct additional tests to assess the extent of the tumor and its effects on brain function. These can include positron emission tomography (PET) scans, which evaluate the metabolic activity of brain tumors and determine their aggressiveness, and electroencephalography (EEG), which assesses the brain’s electrical activity and seizures.
Specific to Barrow Neurological Institute, our Ivy Brain Tumor Center can help oversee more detailed testing to identify a person’s glioblastoma subtype. This additional information helps our neurosurgeons and neuro-oncologists to be even more precise in selecting treatments and clinical trials.
Glioblastoma Treatment
As with all other facets of a brain tumor, optimal glioblastoma treatment depends on the tumor’s type, location, size, and growth rate, as well as a person’s age and overall health. The timing of treatment also influences glioblastoma prognosis—an early diagnosis and surgical intervention are often associated with a better prognosis.
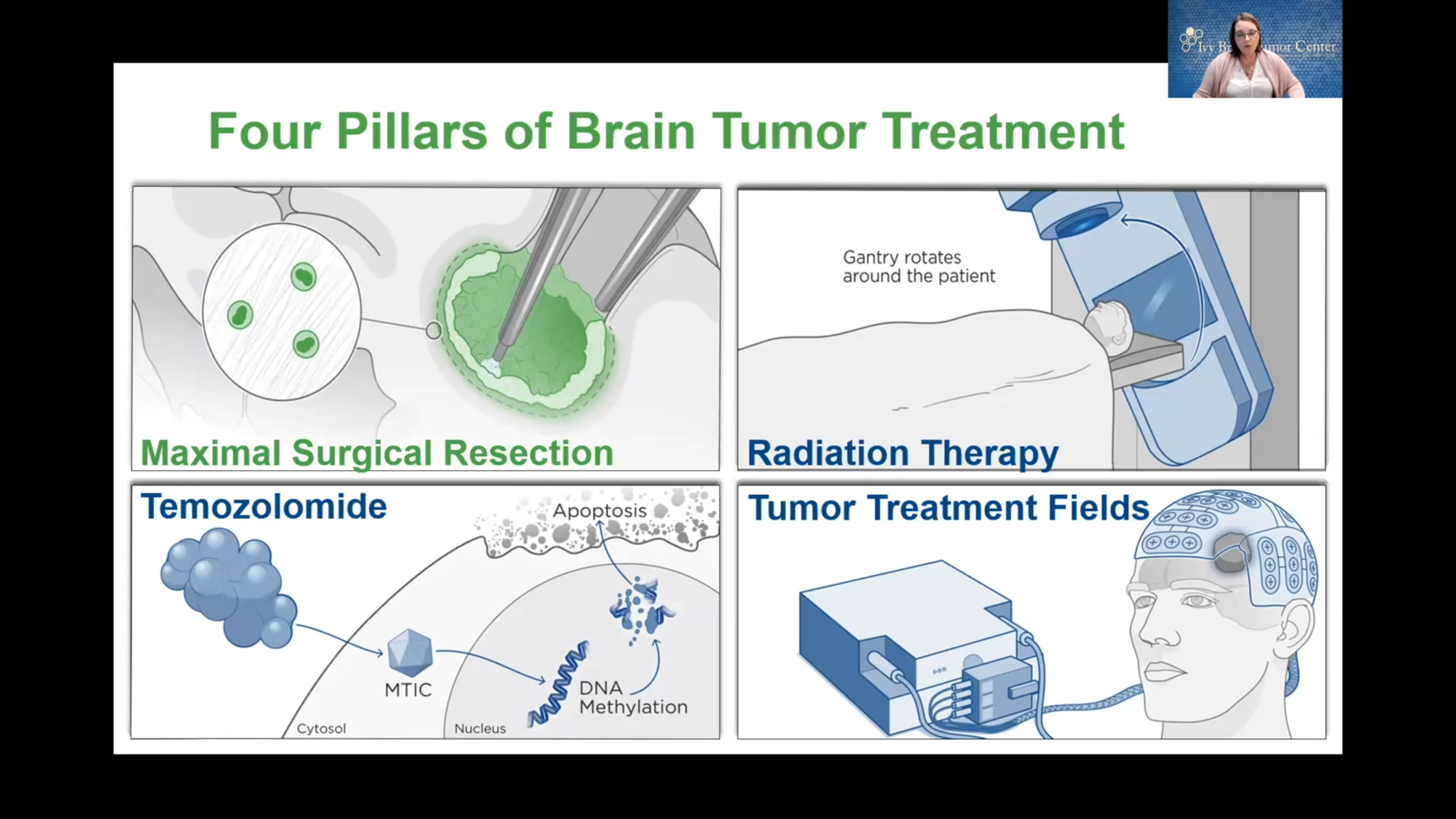
Often, doctors treat glioblastomas with an aggressive combination of surgery, radiation, and chemotherapy. At Barrow Neurological Institute, we offer several advanced options that help surgeons maximize the amount of cancerous tissue removed while sparing as much healthy tissue as possible.

Neurosurgical Treatment
Neurosurgery is often the first step in treating glioblastoma, with the goal of removing as much of the tumor as possible without damaging the surrounding brain tissue. The application of neurosurgery to treat tumors of the brain and spinal cord is called neurosurgical oncology.
Surgery not only reduces the amount of tumor within the brain but also removes cells in the center of the tumor that may be resistant to radiation and chemotherapy while reducing pressure on the surrounding brain. Complete glioblastoma removal is rare due to the tumor’s infiltrative nature and location near critical brain structures.
The two most common surgical procedures for glioblastomas include:
- Craniotomy: A craniotomy is the most common surgery for a brain tumor. During a craniotomy, a neurosurgeon will make an incision in the scalp, remove a portion of the skull, and access the brain to remove as much of the tumor as safely possible while using intraoperative imaging and highly specialized tools. Following surgery, radiation therapy can begin after the surgical wound has healed.
- Awake Craniotomy: Awake brain surgery, or awake craniotomy, is a specialized surgery for brain tumors in areas that control critical functions, like speech or movement. During awake brain surgery, the patient is awake and alert while the neurosurgeon removes as much of the cancerous tissue as possible, allowing the surgical team to monitor brain function in real time and avoid damaging critical brain anatomy.
Radiation Therapy and Radiosurgery
Radiation therapy uses precisely aimed beams of radiation to destroy tumors in the body. While it doesn’t remove the cancer, radiation therapy damages the DNA of the cancerous cells, which then lose their ability to reproduce and eventually die. It’s typically administered after surgery to target any remaining cancer cells in the brain.
Radiosurgery relies on radiation delivery systems to focus radiation at the site of the tumor while minimizing the radiation dose to the surrounding brain. Radiosurgery is often used for tumor recurrence but rarely used in initial treatment for glioblastoma, and the two most common forms are:
- Gamma Knife radiosurgery: This option destroys abnormal tissue through radiation beams focused on a precise tissue area and is only lethal to cells within the immediate vicinity. The Gamma Knife can only be used to treat lesions in the head and involves attaching a metal frame to the skull to target beams of radiation accurately.
- CyberKnife radiosurgery: This technique uses targeted energy beams to destroy tumor tissue while sparing healthy tissue. It uses image-guided robotics to deliver surgically precise radiation to help kill tumors.
Chemotherapy for Glioblastomas
Chemotherapy drugs may be given orally or intravenously to inhibit the growth of cancer cells. Glioblastomas are usually treated with the drug temozolomide, which is typically administered every day during radiation therapy and then given in cycles after radiation. It can be challenging for such drugs to reach the brain due to the blood-brain barrier, which protects the brain from harmful substances but can also block chemotherapy drugs.
Targeted Therapy and Immunotherapy
Targeted therapy drugs specifically target cancer cells by interfering with the molecules involved in tumor growth. These drugs can include those that work to target the genetic mutations commonly found in glioblastoma tumor cells.
Immunotherapy works via a series of drugs that boost the body’s immune system to recognize and attack cancer cells. While this treatment option is still under research, immunotherapy looks promising as a potential treatment for glioblastoma.
Clinical Trials
Glioblastomas are tough opponents because they are combative and can return after treatment. Our scientists and medical professionals constantly seek new ways to mount better odds against glioblastomas. In partnership with the Ivy Brain Tumor Center, Barrow Neurological Institute is proud to be one of the country’s largest sites for neurological clinical trials, with a large and active assortment of glioblastoma clinical trials.
Neuro-Rehabilitation for Quality of Life
At Barrow Neurological Institute, we give our patients access to various neuro-rehabilitation specialists to maximize independence. Neuro-rehabilitation can include physical therapy to help you regain strength and balance, speech therapy to support speaking, expressing thoughts, or swallowing, and occupational therapy to aid you in managing daily activities like bathing, dressing, and using the bathroom. Treating a brain tumor is about more than extending your life—it’s also equally focused on enhancing your quality of life.
One Central Location with Multiple Treatment Options
Glioblastoma surgery is most successful when a highly-skilled neurosurgeon does it—and at Barrow Neurological Institute’s world-class Brain and Spine Tumor Program, we treat people with complex tumors in one robust, full-service location. Our sophisticated multidisciplinary team—neurosurgeons, head and neck surgeons, neuro-oncologists, medical oncologists, and radiation oncologists, to name a few—can offer you the latest treatments for head and neck cancers, including neurological and metastatic cancers.
Finally, we also offer a Brain Cancer Survivorship Program to foster relationships between families affected by brain tumors and provide ongoing support.
Common Questions
How common are glioblastomas?
Glioblastomas represent about 15 percent of primary brain tumors, making them the most common primary brain tumor, or a tumor that begins in the brain versus spreading to the brain from another part of the body.
On average, more than 12,000 glioblastoma cases are diagnosed each year in the U.S., as they’re the most common and aggressive primary brain tumors in adults.
Who gets glioblastomas?
Glioblastomas tend to develop in men more often than in women, in people 50 or older, and in Caucasians. While glioblastomas can occur in children, they’re more commonly diagnosed in older adults—the risk of developing glioblastoma increases with age, with the average age at diagnosis being 64.
What is the prognosis for those diagnosed with glioblastomas?
The prognosis for a person diagnosed with glioblastoma is generally poor, as it’s a highly invasive and difficult-to-treat type of brain tumor.
The median survival time for patients with glioblastoma is around 12 to 18 months from the time of diagnosis, even with aggressive treatment. The disease’s five-year survival rate is around 10 percent. That said, some patients may survive longer if they respond well to treatment, have favorable genetic markers, and have access to innovative therapies through clinical trials.
Despite these known challenges, ongoing research into new treatments, like targeted therapies and immunotherapy, continues to offer hope for improving outcomes in the future.