
The Transseptal Transsphenoidal Approach to Pituitary Tumors
William L. White, MD
Giuseppe Lanzino, MD*
Iman Feiz-Erfan, MD
Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
*Current Address: Department of Neurosurgery, University of Illinois College of Medicine at Peoria, Peoria, Illinois
Abstract
The transseptal transsphenoidal approach allows the surgeon to reach lesions located in the middle of the skull base and is widely accepted as the procedure of choice for the surgical treatment of pituitary adenomas with a primary sellar extension. Despite variations, the procedure consists of a few, well-defined, critical steps, which are summarized and illustrated in this article. The procedure is not only effective but also is well tolerated by patients who can be discharged home 2 or 3 days after surgery with no externally visible scar. More than 700 patients with tumors of the sellar region have been successfully treated by the senior author (WLW) using this technique.
Key Words: macroadenomas, microadenomas, pituitary, transsphenoidal approach
The transsphenoidal procedure as used today was described and successfully performed almost a century ago by bold pioneer surgeons such Cushing and Hirsch. This approach is now widely accepted and considered the procedure of choice for most patients with pituitary tumors. Many subsequent refinements and descriptions have been published.[3,9] This article summarizes and illustrates the critical steps for performing a successful transsphenoidal procedure.
Preoperative Preparation
When the patient is called to the operating room, he or she is administered 4 mg of decadron intravenously and given four sprays of oxymetazoline HCl spray in each nostril. The rest of the bottle of oxymetazoline HCl accompanies the patient to the operating room and is sprayed in both nostrils after the patient is anesthetized and positioned. The patient is placed in a supine position under general intratracheal anesthesia. The throat is packed with a roll of 2-inch gauze. The gauze is tied to the endotracheal tube to ensure its removal before the patient is extubated. The pack prevents operative blood from draining into the stomach and causing postoperative nausea and vomiting.
The head is fixed in a headholder, especially if image guidance is to be used. For reoperations, image guidance is quite helpful in maintaining a midline approach through scar tissue and when the usual landmarks have been removed. We also use image guidance for tumors involving the cavernous sinus and for extended transsphenoidal procedures. We do not use image guidance for routine transsphenoidal procedures and often support the head on a doughnut without skeletal fixation. The latter allows some freedom to make minor adjustments in the patient’s position during the operation to improve the surgeon’s trajectory if desired and avoids the minor discomfort associated with the headholder pins. We prefer to place the head level to slightly flexed and slightly turned to the right. The body is placed diagonally to the left relative to the head. This position allows the surgeon to stand at the patient’s right with a comfortable angle of view. The anesthesiologist is positioned at the patient’s feet. The scrub nurse is to the surgeon’s right and the assistant is to the surgeon’s left. The assistant may move to the patient’s left side to harvest the abdominal fat graft without interrupting the surgeon’s progress.
The eyes are lubricated with petroleum jelly and the eyelids are taped shut. The nostrils are filled with the rest of the bottle of oxymetazoline HCl spray. The face, nose, and mouth are prepared with benzadine scrub and paint. The abdomen is prepared with DuraPrep (3M, St. Paul, MN). The abdominal incision is outlined with a marking pen for the harvest of the abdominal fat graft. Our preferences for the incision site are a previous abdominal incision, and a bikini-type incision for young women. We try to avoid an incision in the right lower quadrant so that the scar is not mistaken for an appendectomy incision. The nose and sublabial incision is draped with four 1000 Steri-drapes (3M Healthcare, St. Paul, MN). The abdominal incision is draped with towels and an Ioban 2 drape (3M Healthcare, St. Paul, MN). Sheets are placed between the two incisions and over the feet. A disposable paper thyroid drape is placed with the opening over the nasal area and a hole cut in the drape for the abdominal incision.
Various approaches and incisions have been used. Regardless of the initial incision and approach (e.g., sublabial, endonasal, endoscopic), the steps are the same once the sphenoid sinus is exposed. The common goal is to reach the pituitary gland and the sella through the sphenoid sinus, maintaining a midline exposure and orientation to these structures. The classic endonasal and sublabial approaches are described below.[3,4,5,9]
Endonasal Exposure
Over the past few years, we have switched from a sublabial to an endonasal approach for most cases. We have found the latter to be somewhat faster and more comfortable for patients. The exposure is slightly smaller but adequate if the surgeon is experienced and comfortable with the transsphenoidal approach. Early in a surgeon’s experience, it may be prudent to use the sublabial approach, which is less likely to deviate from the midline. The sublabial approach also allows a slightly larger exposure with more room for instrument manipulation than the endonasal approach. The small nares of children and some patients may not admit the larger transsphenoidal retractors through one nostril. In this situation, we have used a Killian nasal speculum in place of the transsphenoidal retractor or reverted to the sublabial approach. Care must be taken to avoid tearing the nostril.
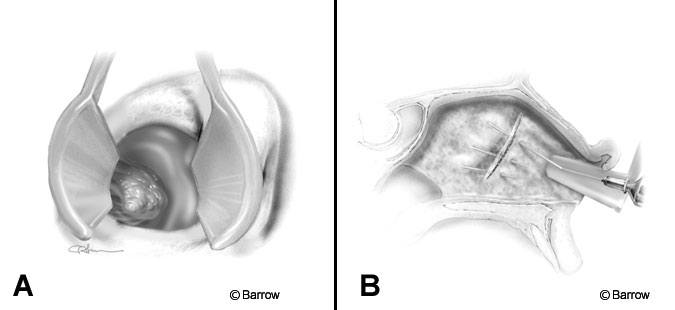
The operating microscope and C-arm are positioned, and magnification is used from the beginning of the endonasal approach (Fig. 1A). The septum and middle turbinate are infiltrated with 0.25% lidocaine and 1:400,000 adrenalin using a 22-gauge, 3-inch spinal needle (Fig. 1B). A 20 degree bend is made in the needle 1 cm distal to the hub, with the needle bevel facing down. This maneuver allows visualization around the syringe. It also allows the needle to be inserted flat along the septum, which improves elevation of the mucosa. Infiltration along the subchondral and subperiosteal plane of the nasal septum is important not only because it decreases bleeding but also because it separates the mucosa from the underlying cartilage and bone, greatly facilitating mucosal dissection later during the procedure.
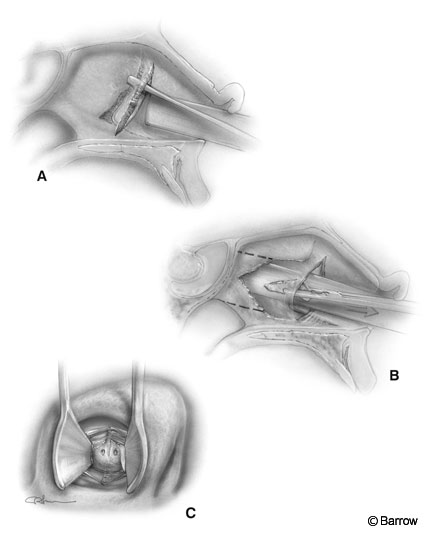
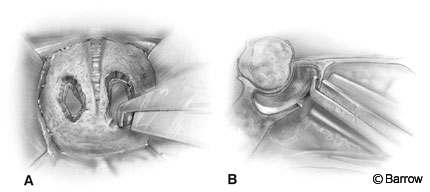
The nostril selected is based on the position of the tumor and the deviation of the septum. The endonasal approach is directed slightly toward the contralateral cavernous sinus. If the tumor extends laterally to one side, it may be advantageous to operate through the contralateral nostril. The nostril selected also is influenced by septal deviations and rarely by the size of the turbinates. Typically, the right nostril is chosen because it is slightly more convenient for a right-handed surgeon if no other factors take priority. We prefer to make a vertical incision approximately halfway back along the septum near the junction of the cartilage and bony septum. The mucosa is detached from the posterior septum (Fig. 2A). The anterior portion of the septum is then fractured or disarticulated from the bony septum. The mucosal dissection proceeds on both sides of the posterior bony septum and along the rostrum (face) of the sphenoid.
The posterior portion of the bony septum is removed and preserved for closure of the sellar floor (Fig. 2B). The rostrum of the sphenoid is widely exposed (Fig. 2B). The nasal speculum is exchanged for the bivalve transsphenoidal retractor and its position confirmed with fluoroscopy. Preferably, the retractor, which tends to drift downward during the procedure, is directed slightly above the sella toward the tuberculum sella. Its direction is confirmed before it is opened to its final position and the turbinates are outfractured. This maneuver decreases the tendency for the retractor to drift.
Sublabial Exposure
The sublabial exposure is usually begun with a headlamp rather than a microscope. The sublabial mucosa is infiltrated with a 1-inch, 25-gauge needle. One side of the septum and the floor of both nostrils are infiltrated using a 22-gauge, 3-inch needle with the needle bent as described for the endonasal approach. Short and long nasal specula are used for retraction.
After the infiltration is completed, a 1- x 3-in cottonoid is placed in each nostril. During the mucosal dissection, visualization of the cottonoid alerts the surgeon to small mucosal perforations so that larger tears can be avoided.
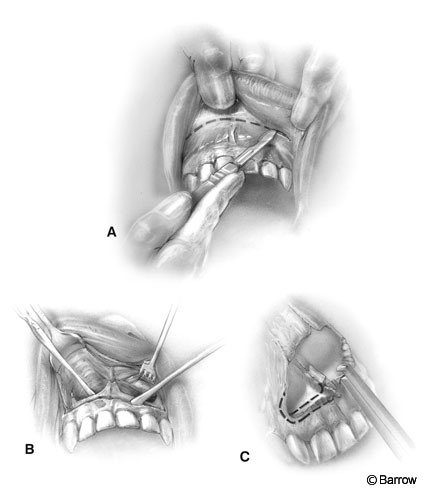
An incision is made 6 to 10 mm superior to the gingival margin (Fig. 3A). Lower incisions jeopardize the neurovascular supply to the teeth with the risk of blackening and necrosis. This incision also leaves a cuff of gingival mucosa to be used for closure.
The incision proceeds down to bone from one canine to its contralateral mate. Subperiosteal dissection of the alveolar process of the maxilla is used to develop a relatively avascular plane. The piriform aperture, including the nasal spine, is exposed.
The dissection continues along the plane of the floor of both nasal cavities. The dissecting instrument should follow the curvature of the maxillary bone forming the floor of the nasal cavity (Fig. 3B). The exposure is enlarged inferiorly by removing the crest of the piriform aperture almost to the level of the floor of the nasal antrum.
The piriform aperture can also be widened with a Kerrison rongeur (Fig. 3C). Excessive removal of the crest may result in exposure and loss of a tooth, and excessive widening may risk injury to the tear duct.
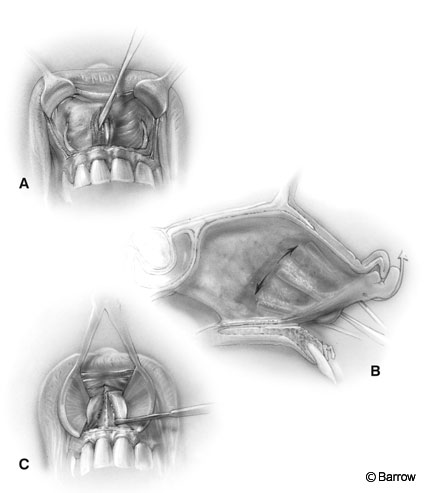
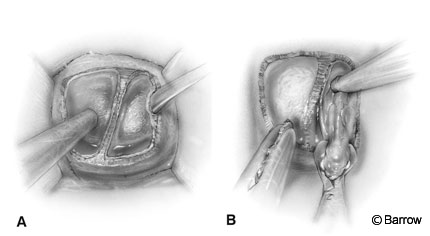
The mucosa is dissected laterally to medially. The attachment of the mucosa to the cartilaginous septum is identified and carefully dissected to preserve the integrity of the mucosa (Fig. 4A and B). It is often tightly adherent to the quadrangular cartilage at the junction of the floor.
The mucosa is left attached to one side of the anterior nasal septum (usually to the left). This maneuver protects the septum and reduces the postoperative incidence of anterior septal perforations.
The exposed quadrangular cartilage is disarticulated from the vomer and the anterior aspect of the perpendicular ethmoidal plate, and a long nasal speculum is inserted for retraction (Fig. 4C). The anterior septum is displaced laterally into one nostril (usually the left). This maneuver creates a tunnel on one side (usually the right) and the septum with the mucosa attached to it on the other side (usually the left).
The anterior edge of the vomer and the perpendicular plate, which have been disarticulated from the quadrangular cartilage, are identified. The mucosa is dissected free posteriorly from each side of the bony septum and the rostrum (face) of the sphenoid. The nasal speculum is replaced with a bivalve transsphenoidal retractor.
The operating microscope and C-arm image intensifier are positioned, and the direction of the retractor is confirmed. The middle turbinates are outfractured if necessary. After this point the rostrum of the sphenoid sinus is well exposed and the steps involved are the same as with the endonasal approach.
[one_half]
[/one_half]
[one_half_last]
[/one_half_last]
Exposure of the Sphenoid Sinus
After the rostrum of the sphenoid sinus has been exposed, the sphenoid ostia are identified. A 2-mm, 45 degree Kerrison rongeur is introduced through the ostia, and the rostrum of the sphenoid sinus is removed (Fig. 5). The bone that is removed may be preserved to supplement the closure. The septum within the sphenoid sinus is identified and compared to the patient’s preoperative contrast magnetic resonance (MR) images or, if available, computed tomographic (CT) scans, because the septum provides a valuable landmark for orientation. The septum is then removed with pituitary rongeurs. Removal of the sphenoid mucosa (Fig. 6) reduces the incidence of postoperative hemorrhage and mucocele formation. The sellar floor is identified and confirmed with fluoroscopy. The appearance of the sella is determined by the extent of pneumatization of the sphenoid sinus and should be anticipated by noting the extent of the pneumatization on the patient’s preoperative radiologic studies.
The floor of the sella is often eroded or thinned by tumor, especially in the case of macroadenomas, and is easily removed. In the case of microadenomas, the floor of the sella tends to maintain its normal consistency. It is then opened cautiously with a chisel and a mallet or a power drill using a diamond burr. During this maneuver, care must be exercised to avoid compromising the underlying dura.
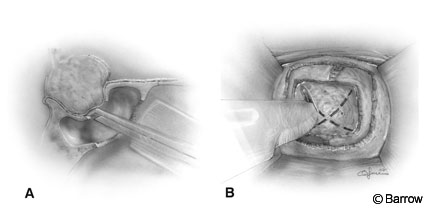
The opening is completed and enlarged using Kerrison rongeurs (Fig. 7A). Wide exposure of the sellar floor laterally to the carotid recesses and cavernous sinuses and anteriorly to the circular sinus is a must in the case of macroadenomas. Bleeding is controlled with hemostatic agents, Gelfoam (Upjohn Company, Kalamazoo, MI) and thrombin or FloSeal (Fusion Medical Technologies, Baxter Healthcare Corporation, Freemont, CA) and tamponade with cottonoids. Bleeding from the cavernous sinus can be controlled by inserting small pieces of Gelfoam and thrombin into the cavernous sinus. Bone wax can be squeezed gently against the bleeding bony edges by using a microcottonoid. Occasionally, the tumor must be removed in a bloody field and the bleeding controlled later. Usually, however, the tumor can be removed more completely and safely if time is taken to control the bleeding before and during the dural opening. Before the dura is opened, cauterization is avoided because it can discolor the underlying gland and tumor, making identification of the tumor more difficult.
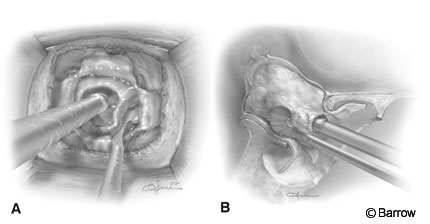
Once the dura has been exposed adequately under the microscope, the dura is opened in a stellate fashion with a No. 11 or No. 15 blade and curved microscissors (Fig. 7B). Caution is necessary when incising the dura to avoid damaging a possibly ectatic carotid artery, which may be located within the sella. When the dura is opened, immediate attention is directed to differentiating normal gland from tumor. Frequently with large tumors, the only evidence of residual gland may be a very thin, arachnoid-like layer covering the tumor. This layer can be preserved. As tumor is removed, this layer retracts cephalad, gradually becoming thicker and more obviously normal pituitary gland. Large tumors are entered inferiorly, biopsied, and minimally debulked centrally while preserving the margins and avoiding the superior aspect of the tumor (Fig. 8). The inferior and lateral rim of the tumor is then carefully separated from the dura and normal gland with enucleators. The enucleators function best by using the backside of the blade to nudge the lateral rim of tumor into the sella (Fig. 8). The plane between the tumor and the dura is usually established along the floor of the sella. The down blade is used to dissect the left rim of the tumor. The up blade is used to dissect the right rim of the tumor and is inserted upside down, beginning at the tumor-dura interface along the floor of the sella. The dissection gradually proceeds laterally. The posterior portion of the tumor is removed from the region of the clivus before an anterosuperior extension is addressed. This strategy allows the tumor to tilt backward and the anterior diaphragm to push the anterior suprasellar portion of the tumor into the sella. It helps prevent the diaphragm from collapsing into the sella in front of the posterior portion of the tumor and obstructing visual access to a suprasellar nodule.
The delivery of the tumor is facilitated by normal intracranial pressure (ICP). Lumbar drainage, which decreases ICP, is not routinely used. If a cerebrospinal fluid (CSF) leak occurs or CSF is drained, the tumor, diaphragm, and pituitary will rise into the intracranial cavity and will not be easily accessible through the usual transsphenoidal approach. After the inferior, lateral, and posterior portions of the tumor have been separated and their delivery has begun, the superior margin is carefully dissected.
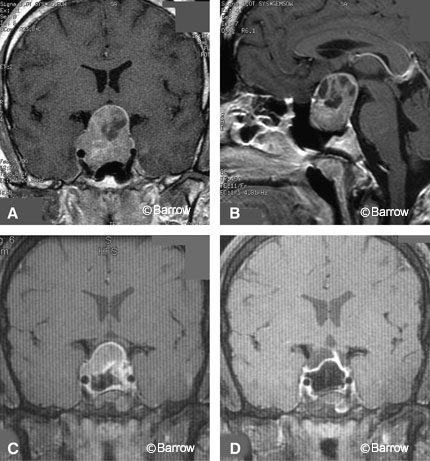
The residual pituitary gland usually adheres to the diaphragm and is preserved when possible. Sometimes the tumor pushes the pituitary gland and stalk to one side, which may be predicted by the deviation of the stalk on MR imaging. Ring curettes are not used early in the procedure. They are seldom used and only after dissection with the enucleators is completed. The long arc, smooth, small ring curettes are used to gently nudge lateral and superior tumor extensions that are beyond the reach of enucleators. Hollowing the tumor with ring curettes tends to leave a rind of tumor that can be difficult to deliver. All instruments are used gently by applying little more pressure than the weight of the instrument. Biopsies and tumor removal with a micropituitary rongeur should present no resistance. If resistance to a biopsy is felt, the specimen should be released as its removal will likely be followed by CSF. Pituitary tumors are rarely fibrotic (as is often encountered in recurrent tumors, tumors that secrete thyroid stimulating hormone, or tumors treated long-term with bromocriptine) and therefore are rarely difficult to remove. Removing the tissue that adheres to the diaphragm (usually normal pituitary gland) will cause a CSF leak, diabetes insipidus, and hypopituitarism. It is prudent to err on the side of conservatism if the optic chiasm is adequately decompressed in patients whose vision is compromised. Diabetes insipidus usually resolves with time if traction has not been applied to the pituitary stalk, injuring the hypothalamus.
For large tumors, a staged transsphenoidal resection may be planned.[8] The advantage of a staged procedure is that the tumor tissue remaining after the first resection can settle downward into the sella, facilitating gross total resection (Fig. 9).
Recognition of the normal anterior lobe, pars intermedia, and posterior lobe; of cavernous sinus invasion; and of the many different appearances and textures of various pituitary lesions is acquired by experience. Experience is especially necessary to recognize and remove microadenomas. Recognizing CSF leaks is crucial to designing an adequate closure. CSF may appear as a black oozing within venous blood, or it may be detected after the anesthesiologist performs a Valsalva maneuver. Postoperative lumbar drainage is advisable if there is significant concern about postoperative CSF leakage.
Closure
If a CSF leak occurs or there is concern about the potential for herniation of the chiasm into the sella, an abdominal fat graft is harvested. A portion of the fat is sized and placed within the tumor bed. If a large defect is present in the diaphragm, fascia can also be used to supplement the diaphragm.
The bone or cartilage salvaged from the septum and sphenoid rostrum or, if inadequate, a piece of MacroSorb PS bioabsorbable plate (MacroPore Inc., San Diego, CA) or cortical strip of allograft bone may be used to reconstruct the sellar floor. The implant is cut slightly wider than the sellar opening and is wedged extradurally to cover the dural opening. If there is significant concern about a CSF leak, the sellar opening may be covered with a thin layer of Tisseel (Baxter Healthcare Corporation, Deerfield, IL). Typically, we cover it with a piece of Gelfoam soaked in thrombin.
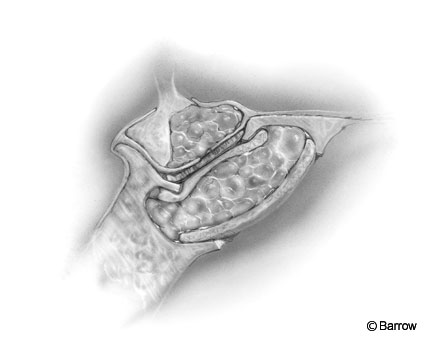
The sphenoid sinus is filled with fat. If fat has not been harvested to use in the sella, sphenoid sinus bleeding is controlled with Gelfoam soaked in thrombin and is left in place. The transsphenoidal retractors are removed (Fig. 10).
The endonasal approach is closed by repositioning the septum mucosa over the sphenoid osteotomy and against the mucosa from the contralateral side. This maneuver re-forms the face of the sphenoid and the posterior septum. The turbinate is elevated. A small piece of Gelfoam is placed under the turbinate to support it, preventing adhesions and obstruction of the maxillary sinus drainage. A nasal tampon soaked in Mycolog cream (Bristol-Myers Squibb, Princeton, NJ) is placed in the nostril, supporting the mucosal flap against the midline and directed toward the mucosal os. The contralateral nostril is then explored, irrigated, and packed with a nasal tampon, repositioning the septum to the midline. The nasal tampons are tied together, and a sterile mustache dressing is applied.
After a sublabial approach, the transsphenoidal retractor is removed, the wound is irrigated, and bleeding is controlled. The mucosa is investigated for holes or tears. If large holes are present in the mucosa, especially if they are anterior, bilateral and adjacent to each other, they are closed (at least on one side) with 4-0 chromic suture. If they are unilateral, small, and posterior, they do not require closure. Large anterior opposing holes will result in an anterior septal defect that may become symptomatic. The sublabial incision is closed with a 4-0 running chromic suture. The nostrils and nasopharynx are explored and irrigated. If necessary, the turbinates are elevated and supported with a small piece of Gelfoam. The nasal airways are packed with nasal tampons soaked with Mycolog cream. The tampons are tied together, and a sterile mustache dressing is applied. Lumbar drainage may be instituted if there is a concern about CSF leakage but is rarely necessary for a routine transsphenoidal procedure. The nasal packs are removed the first or second day after surgery, and the patient is discharged the second or third day after surgery.
At the onset of surgery, 1.5 gm of cefuroxime is given intravenously, and a second dose is given 8 hours later and then discontinued. When CSF leaks are a concern, the head of the bed is elevated 30° for 10 days. A Foley catheter is maintained overnight for monitoring urine output hourly. The patient is observed in the intensive care unit overnight. An ice bag is placed over the nose and upper lip. Antiembolism stockings are used until the patient is ambulating. Steroid replacement therapy is continued until the patient’s reserve is confirmed to be adequate. Sodium is monitored every 6 hours for 36 hours, then every 8 hours for 24 hours, and then daily.
Endoscopic Surgery
Endoscopic surgery has some attractive theoretical advantages, and some surgeons advocate a completely endoscopic procedure.[1,6] We have adopted an endoscopic-assisted approach utilizing the endoscope to visualize the tumor cavity usually after microsurgical excision of the tumor. We have employed 4-mm, 0 degree, 30 degree and, occasionally, 60 degree endoscopes (Storz [Karl Storz Endoscopy, Culver City, CA] or Aesculap [San Francisco, CA]) to assist in microsurgical procedures. An endoscope can help reveal laterally invasive residual tumors. We have found that using the endoscope in conjunction with the Zeiss Multivision (Carl Zeiss, Thornwood, NY) component to be helpful in inserting the endoscope. The Multivision component allows visualization of the tip of the endoscope within the operative site by the left operating microscope lens while monitoring the endoscopic view through the right lens. The instrumentation and endoscopes are evolving, and continued investigation of the endoscopic approach is warranted.
Conclusion
Transsphenoidal surgery is well tolerated. Results can be excellent with minimal complications, but surgeons must have a thorough knowledge of the surrounding anatomy and potential complications.[2,7,10] Experience is essential and is best acquired when institutions limit transsphenoidal surgery to one surgeon. The neurosurgeon also must thoroughly understand pituitary endocrinology to select patients for surgery, to manage pre- and postoperative care, and to make appropriate decisions during surgery.
References
- Alfieri A, Jho HD: Endoscopic endonasal cavernous sinus surgery: An anatomic study. Neurosurgery 48:827-837, 2001
- Ciric I, Ragin A, Baumgartner C, et al: Complications of transsphenoidal surgery: Results of a national survey, review of the literature, and personal experience. Neurosurgery 40:225-237, 1997
- Coldwell WT, Weiss MH: Transnasal transsphenoidal approach, in Apuzzo MLJ (ed): Surgery of the Third Ventricle. Baltimore: Williams and Wilkins, 1998, pp 553-574
- Hardy J: Transsphenoidal microsurgery of the normal and pathological pituitary. Clin Neurosurg 16:185-217, 1969
- Hardy J: Transsphenoidal hypophysectomy. J Neurosurg 34:582-594, 1971
- Jho HD, Alfieri A: Endoscopic endonasal pituitary surgery: Evolution of surgical technique and equipment in 150 operations. Minim Invas Neurosurg 44:1-12, 2001
- Laws ER, Kurn EB: Complications of transsphenoidal surgery, in Tindall GT, Collins WF (eds): Clinical Management of Pituitary Disorders. New York: Raven Press, 1979, pp 435-445
- Mohr G, Hardy J, Comtois R, et al: Surgical management of giant pituitary adenomas. Can J Neurol Sci 17:62-66, 1990
- Pappas CTE, White WL, Baldree ME: Pituitary tumors: Anatomy, microsurgery, and management. BNI Quarterly 6(4):2-12, 1990
- Semple PL, Laws ER, Jr.: Complications in a contemporary series of patients who underwent transsphenoidal surgery for Cushing’s disease. J Neurosurg 91:175-195, 1999
