Department of Neurosurgery
Our Mission
The faculty physicians in the Department of Neurosurgery at Barrow Neurological Institute in Phoenix, Arizona operate in 11 neurosurgery-dedicated, technologically advanced operating theaters. Neuroanesthesiology at Barrow complements neurosurgery by providing comfort, immobilization and sophisticated monitoring of the patient’s neurological functions. One such procedure is “cardiac standstill,” pioneered at Barrow to repair difficult aneurysms by lowering body temperature, stopping the heart and recirculating the blood during delicate operations.
Barrow is proud of its reputation as a medical research facility and is home to some of the most sophisticated research programs outside of an academic setting in the Southwestern United States. Researchers in the Barrow Neurosurgery Research Center are devoted to learning more about the causes of and treatments for a wide range of disorders, such as stroke, aneurysms, spinal cord injury, and hydrocephalus. Our research has led to treatments that are being used around the world. Within the Department are neurosurgeons who subspecialize in cerebrovascular and skull base, functional and stereotactic, pediatric, spine, tumor, and endovascular neurosurgery.
Physicians, scientists, and expert clinical staff come together with a commitment to developing new techniques for the prevention, diagnosis, treatment, and rehabilitation of neurological illnesses and injuries. The leadership of internationally respected physicians keeps Barrow at the forefront of neuroscience. The compassionate expertise of its extensive staff of nurses, technologists, therapists and support personnel is evident in every aspect of care provided.
Our department is chaired by Michael T. Lawton, MD.
Specialists
Faculty

Stephen Papadopoulos, MD
Neurosurgery
Fellows
Residents
Research
This study will look at how well 5-ALA, an amino acid compound, can identify cancer cells during surgery and improve tumor removal in patients with high- and low-grade gliomas. Select patients take an oral dose of either 5-ALA or ascorbic acid a few hours before...
This study is being done to find which dose of 5-ALA is the best for seeing recurrent gliomas during surgery. Select patients will take an oral dose of study drug (5-ALA) a few hours before surgery. During the procedure surgeons use a special microscope that...
The study will include AVM patients and members of their nuclear family. All patients will donate a sample of saliva (5 mL) or blood (10 mL). Basic demographic and clinical parameters will be collected, including ethnicity, age at first presentation, symptoms at presentation, history of...
Prevention of stroke involves managing and treating risk factors. Most strokes are caused when blood flow to a portion of the brain is blocked. One place this often happens is in the carotid artery. This blockage is called atherosclerosis or hardening of the arteries. The...
This is a randomized, active-controlled, multicenter, open-label, parallel groups, Phase 2 study of DSP-7888 Dosing Emulsion plus Bevacizumab versus Bevacizumab alone in patients with recurrent or progressive glioblastoma multiforme (GBM) following treatment with first line therapy consisting of surgery and radiation with or without chemotherapy....
In the proposed trial, patients will be administered ribociclib+everolimus prior to surgical resection of their tumor. Recurrent GBM patients will be randomized into one of the three time-interval cohorts. All patients will receive ribociclib 400 mg and everolimus 2.5 mg (R2P2D) orally-administered in 5 daily...
In the proposed trial, patients will be administered ribociclib prior to surgical resection of their tumor. Patients will be enrolled in time-intervals sequentially (non-randomized). All patients will be orally-administered 5 doses of LEE011 (900 mg/d) with the final dose occurring at one of 3 intervals...
This trial is an open-label, multicenter, Phase 0/2 trial that will enroll up to 50 participants with recurrent glioblastoma which are schedule for resection. In the lead-in cohort, a total of 10 participants will be enrolled into the proposed phase 0 clinical trial. Participants will...
This trial is an open-label, multicenter, Phase 0 trial that will enroll up to 20 participants with recurrent high-grade glioma with FGFR1 K656E or FGFR3 K650E mutation or FGFR3-TACC3 translocation which are scheduled for resection. In the lead-in cohort, a total of 20 participants will...
This is an open-label, single-center Phase 0/2 study that will enroll up to 30 participants with newly diagnosed (N=12) and recurrent glioblastoma (N=18). The trial will be composed of a Phase 0 component (subdivided into Arm A, Arm B, and Arm C), and an Exploratory Phase...
A Phase 0 single center, first in human, open-label study of ascending energy doses of sonodynamic therapy (SDT) utilizing the MRgFUS combined with intravenous ALA to assess safety and efficacy in up to 30 participants with recurrent HGG. Eligible participants who are scheduled for resection will...
In the proposed trial, patients will be administered ribociclib prior to surgical resection of their tumor. Patients will be enrolled in time-intervals sequentially (non-randomized). All patients will be orally-administered 5 doses of LEE011 (900 mg/d) with the final dose occurring at one of 3 intervals...
In the proposed trial, patients will be administered ribociclib+everolimus prior to surgical resection of their tumor. Recurrent GBM patients will be randomized into one of the three time-interval cohorts for the first two dose levels. In the lead-in dose escalation study, the first six subjects...
This is an open-label, multi-center Phase 0 study with an expansion phase that will enroll up to 24 participants with newly-diagnosed glioblastoma and up to 18 recurrent glioma participants with IDH mutation and ATRX loss. The trial will be composed of a Phase 0 component...
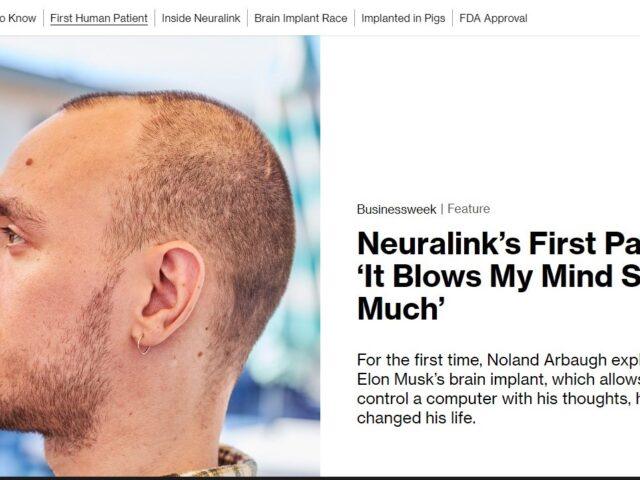
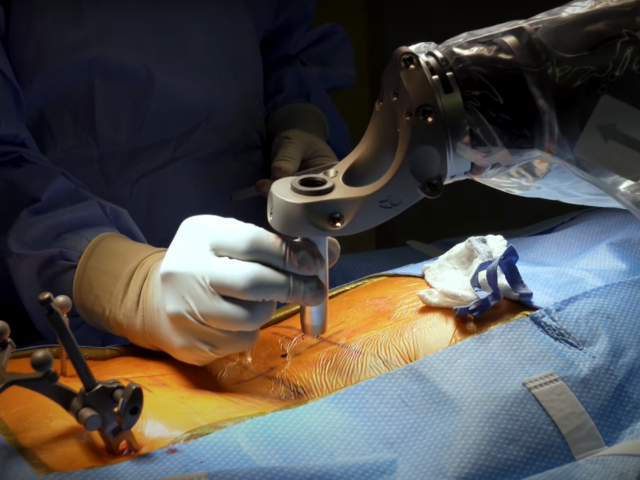
PRIME Study: Precise Robotically Implanted Brain-Computer Interface If you have tetraplegia due to spinal cord injury or ALS and want to explore new ways of controlling your computer, we invite you to participate in Neuralink’s clinical trial. Participants will go through a robot-assisted surgery to...