
Ascites after Ventriculoperitoneal Shunting Associated with Elevated Levels of CSF Protein
Authors
Hans G. Eder, MD†
Wilfried Gruber, MD‡
Klaus A. Leber, MD†
Karin Bernard, MD†‡
Harold L. Rekate, MD
Division of Neurological Surgery, Barrow Neurological Institute, Mercy Healthcare Arizona, Phoenix, Arizona
†Department of Neurosurgery, ‡Department of Pediatrics, University of Graz, Austria, †‡Zentrum fur Blut- und Krebserkrankungen, Berlin, Germany
Abstract
A 5.5-year-old girl developed cerebrospinal fluid (CSF) ascites after ventriculoperitoneal (VP) shunting. The VP shunt was placed to treat non communicating hydrocephalus caused by an optic glioma. There was no evidence of CSF or peritoneal infection or tumor seeding into the peritoneal cavity. The ascites completely resolved after the shunt was converted to a ventriculoatrial system. The CSF as well as the ascites fluid contained elevated protein concentrations (375 mg/dl and 1600 mg/dl, respectively). The results of electrophoresis suggest that the severely elevated levels of CSF protein reflected a disturbed blood-brain barrier. These elevated levels of protein then likely led to the failure of peritoneal absorption of the CSF.
Key Words : ascites, blood-brain barrier, electrophoresis, hydrocephalus, optic glioma, ventriculoperitoneal shunt
Of all pediatric neurosurgical procedures, cerebrospinal fluid (CSF) shunts carry the highest incidence of complications.8 CSF ascites after ventriculoperitoneal (VP) shunting, however, is rare: We have found only 22 published reports of this complication. In seven cases, the CSF ascites was associated with elevated levels of CSF protein and a brain tumor (six optic gliomas and one craniopharyngioma).
Different etiologic factors underlying the development of ascites after VP shunting have been proposed, including the possibility of a defective blood-brain barrier (BBB). We report another patient with anoptic glioma who developed ascites after VP shunting for hydrocephalus. The results of CSF protein electrophoresis suggest that this unusual association of optic gliomas with ascites may reflect a disturbance of the BBB that permits the concentration of protein in the CSF to reach excessive levels.
Case Report
At the age of 6 months, a Caucasian girl was admitted to a children’s hospital elsewhere for evaluation after experiencing nystagmus for 4 weeks. Computed tomography and magnetic resonance (MR) imaging revealed a large tumor (5 x 5 x 5 cm) in the region of the optic chiasm. She underwent immediate surgery for resection, and two-thirds of the tumor and a part of the right optic nerve were removed. Histological examination revealed a low-grade astrocytoma (WHO grade I). No criteria for neurofibromatosis were identified.
A routine follow-up MR imaging study demonstrated rapid growth of the initially small remaining tumor to 4 x 3 x 3 cm. At the age of 16 months, she underwent another operation at the same institution and about50% of the tumor was removed. After the operation, she underwent external beam radiation (40 Gy). Subsequent MR imaging revealed renewed tumor growth that led to a third operation at the same institution when the girl was 4years and 8 months old. This operation was performed on the basis of the MR imaging study; no new neurological deficits were reported. At that time, the vascularity of the tumor allowed only a limited resection. The outcome of the histological examination was again the same. Postoperative MR imaging showed hydrocephalus due to an obstruction of the foramen of Monro. Shunting was performed with bilateral ventricular drains connected proximal to the valve using a “Y” connector and draining to the peritoneal cavity.
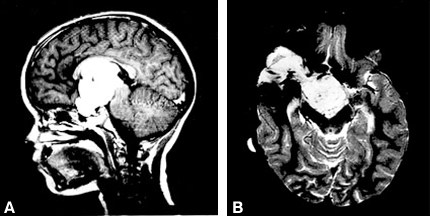
Nine months later the girl presented to the Department of Neurosurgery, University of Graz, Austria, because the circumference of her abdomen had increased in the previous 2 months. On admission, her general medical condition was good and she was afebrile. The size of her liver and spleen was normal as was an internal examination. A neuro ophthalmologic examination revealed convergent strabismus, amaurosis of the right eye, and temporal hemianopsia of the left eye. All serum parameters, including liver function tests, were within normal ranges. Abdominal sonography revealed severe ascites with signs of elevated protein levels in the intraperitoneal fluid and confirmed that the liver, parenchymal structure, and spleen were normal. Doppler sonography indicated no portal hypertension. A new MR imaging study showed that the tumor had progressed (Fig. 1) compared to her earlier studies (not available).
CSF from the shunt reservoir was xanthochromic and had an increased number of leucocytes (64/mm3) and erythrocytes (6930/mm3). The serum sediment reflected 44% lymphocytes, 42% polymorphs, and 14% monocytes. Tumor cells were absent. The level of total CSF protein was excessive at 375 mg/dl (compared with the normal value(NV) of 11.5 to 35.5 mg/dl for this age group).12 CSF protein electrophoresis demonstrated increased absolute concentrations of all protein fractions with significant displacements of relative fractions. At 75.1%albumin (NV: 61 to 73%) was increased while a2-globulin at 6.5% (NV: 4.4 to 7.6%), b1-globulin at 5.3% (NV: 5.9 to 9.9%) and g-globulinat 4.8% (NV: 5.3 to 8.4%) were normal or only slightly reduced. Prealbumin at 0.4% (NV: 4.3 to 8.7%) and t-globulin at 1% (NV: 1.8 to 5.3%) were considerably reduced.12In addition, a hemoglobin fraction was discovered. The IgG-index [quotient IgG (CSF/serum): quotient albumin (CSF/serum)] of 0.65 was within the reference range of < 0.7.15 A CSF culture was sterile.
Ascitic fluid was also xanthochromic and had an increased number of leucocytes (96/mm3) and erythrocytes (1120/mm3) as well as an excessively elevated level of protein (16,000 mg/l). Protein electrophoresis of the ascitic fluid revealed the following relative values: pre albumin, 0.1%; albumin, 77.4%;a2-globulin, 5.9%; b1-globulin, 5.9%; t-globulin, 0.6%; and g-globulin, 5.5%. The ascitic sediment showed some lymphocytes and monocytes, several macrophages and erythrophages but no tumor cells. The ascitic culture also was sterile.
After all examinations were complete, the VP shunt was converted to a ventriculoatrial (VA) systemand the ascites regressed.
Discussion
Shunt procedures may be necessary in patients with an optic glioma who develop ventriculomegaly from an obstruction of the anterior third ventricle and foramen of Monro2 caused by either mass effect or the tumor itself. However, cases of communicating hydrocephalus also have been reported.13,16,17 Our patient suffered from obstructive hydrocephalus, which made VP shunting necessary.
The development of ascites after the implantation of a VP shunt is a rare complication. The literature on cerebral tumors associated with CSF ascites and VP shunting suggests several etiologic factors as the cause of the ascites. For example, peritoneal metastases might be the primary factor underlying ascites associated with medulloblastomas6 and pineoblastomas.3,11 In the case of choroid plexus papilloma, ascites has been attributed to a massive overproduction of CSF.9 Adegbite and Khan1 first reported a high CSF-protein content as the predisposing factor for the development of ascites associated with VP shunting of cerebral tumors. Their patient had a craniopharyngioma and elevated CSF-protein levels and developed ascites after a VP shunt was implanted. The VP shunt was changed to a VA system. The ascites resolved completely, and CSF-protein levels returned to normal after the tumor was resected. Protein electrophoresis was not performed.
Six patients with optic gliomas and shunted hydrocephalus also have had elevated CSF-protein levels that were believed to have caused ascites.13,16,17 The course of the patient treated by Tanget al.13 was similar to that of our patient in terms of protein levels in the CSF and the necessity of converting from a VP to a VA shunt. West et al.16 reported another three of these six patients. Inone of their three patients, the optic glioma stopped growing after radiation treatment and the level of protein in the CSF decreased. Yount et al.17 treated two patients. Their ascites protein levels were high but their CSF-protein levels were increased only slightly (less than 100 mg%). In both patients the ascites resolved spontaneously and without surgery, leading the authors to assume that multiple factors were involved. Again, protein electrophoresis was not performed in any of these cases.
Although ascites is a risk in children with optic gliomas, VP shunting is still the procedure of choice. The complication is rare and can easily be resolved by a VP to VA shunt conversion.
In our case, the results of the CSF-protein electrophoresis suggest that a disturbed BBB ledto the elevation of both CSF and ascitic fluid. The distribution of CSF-protein fractions demonstrates increased penetration of plasma proteins into the CSF. Characteristic features are the decreased concentration of pre albumin and t-globulin and the more prominent representation of albumin in the electrophoresis. t-protein is synthesized in the central nervous system (CNS) and prealbumin in both the liver and the CNS.4,14 When the BBB is intact, the concentrations of both proteins are higher in the CSF than in the serum. If the BBB is disturbed, the dilution of the plasma reduces the concentrations of both fractions in the CSF. An increase in the concentration of albumin, which is only synthesized in the liver, would most likely indicate increased penetration of macromolecules into CSF from the blood. Tumors can also produce proteins; however, it is unlikely that any protein released by the glioma would match the electrophoretic pattern of the albumin. Furthermore, the absorption of CSF within the abdominal cavity, which mainly occurs via the lymphatic vessels in the diaphragm, would be impaired by elevated levels of protein in the CSF.5,10
The electrophoresis revealed a hemoglobin fraction in the CSF and ascites. This fraction and the xanthochromic CSF indicated the presence of an older hemorrhage. An intrathecal immune reaction was ruled out by the normal level of IgG, which was within the reference range according to Tibbling.15 This assumption is supported by the absence of eosinophils in the fluid. The large surface area of the optic glioma combined with its extreme vascularity may have attributed to the breach of the BBB and hence to the increased concentration of protein.
Whether the BBB might be disturbed within the normal brain or within the optic glioma itself is also speculative. The breakdown of the BBB could reflect either new vessels within the tumor that have not assumed the properties of the normal BBB, or the properties of existing vessels could have changed. Indeed, cultured neoplastic glial cells produce a permeability factor that is capable of opening the BBB in vivo.7 Perhaps a similar mechanism could operate in nonmalignant but fast-growing tumors. In any event, that ascites has developed after VP shunting in only eight reported cases of optic gliomas, which is a relatively common tumor, suggests unusual conditions prevail in these patients. It would be useful if future reports noted the relative vascularity of optic gliomas or other tumors associated with high levels of protein in the CSF to confirm this observation.
References
- Adegbite AB, Khan M: Role of protein content in CSF ascites following ventriculoperitoneal shunting. Case report. J Neurosurg 57:423-425, 1982
- Alvord EC, Jr., Lofton S: Gliomas of the optic nerve or chiasm. Outcome by patients’ age, tumor site, and treatment. J Neurosurg 68:85-98, 1988
- Cranston PE, Hatten MT, Smith EE: Metastatic pineoblastoma via a ventriculoperitoneal shunt: CT demonstration. Comput Med Imaging Graph 16:349-351, 1992
- Herbert J, Wilcox JN, Pham KT, et al: Transthyretin: A choroid plexus-specific transport protein in human brain. The 1986 S. Weir Mitchell Award. Neurology 36:900-911, 1986
- Lill SR, Parsons RH, Buhac I: Permeability of the diaphragm and fluid resorption from the peritoneal cavity in the rat.Gastroenterology 76:997-1001, 1979
- Mori T, Kayama T, Katakura R: Medulloblastoma with intractable ascites treated by carboquone.A complication of a ventriculoperitoneal shunt [Japanese]. No Shinkei Geka 5:1299-1303, 1977
- Ohnishi T, Sher PB, Posner JB, et al: Capillary permeability factor secreted by malignant brain tumor. Role in peritumoral brain edema and possible mechanism for anti-edema effect of glucocorticoids. JNeurosurg 72:245-251, 1990
- Pople IK, Quinn MW: Morbidity and outcome of shunted hydrocephalus. Z Kinderchir 45:29,1990
- Ray BS, Beck FC, Jr: Papilloma of the choroid plexus of the lateral ventricles causing hydrocephalus in an infant. J Neurosurg 13:405-410, 1956
- Raybuck HE, Allen L, Harms WS: Absorption of serum from the peritoneal cavity. Am J Physiol 199:1021-1024, 1960
- Saibara T, Hashimoto T, Takahashi M, et al: Abdominal metastasis of a pineal region tumor through ventriculoperitoneal shunt. Case report [Japanese]. Neurol Med Chir (Tokyo) 31:1012-1017, 1991
- Siemes H, Siegert M, Rating D: The relationship between age and the cerebrospinal fluid protein profile of normal children. Cellulose acetate and agarose gel electrophoretic studies [German]. Neuropädiatrie 6:383-397, 1975
- Tang TT, Whelan HT, Meyer GA, et al: Optic chiasm glioma associated with inappropriate secretionof antidiuretic hormone, cerebral ischemia, nonobstructive hydrocephalus and chronic ascites following ventriculoperitoneal shunting. Childs Nerv Syst 7:458-461, 1991
- Thompson EJ, Keir G: Laboratory investigation of cerebrospinal fluid proteins. Ann ClinBiochem 27:425-435, 1990
- Tibbling G, Link H, Ohman S: Principles of albumin and IgG analyses in neurological disorders.I. Establishment of reference values. Scand J Clin Lab Invest 37:385-390, 1977
- West GA, Berger MS, Geyer JR: Childhood optic pathway tumors associated with ascites following ventriculoperitoneal shunt placement. Pediatr Neurosurg 21:254-259, 1994
- Yount RA, Glazier MC, Mealey J, Jr., et al: Cerebrospinal fluid ascites complicating ventriculoperitoneal shunting. Report of four cases. J Neurosurg 61:180-183, 1984
