
Alzheimer’s Disease: Clinical Diagnosis and Treatment
Patricio F. Reyes, MD
Lee A. Nowak, MS
S. Gabe Rice, MS
Division of Neurology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Alzheimer’s disease is an insidious disease with staggering social, economic, and emotional effects on both loved ones and society. The greatest risk factor of AD is age, and as the population of elderly increases, the burden of AD will also increase. There is no cure and the best medical treatments only manage symptoms. Knowledge gained from basic science and clinical research improves our understanding of AD and associated risk factors. Early diagnosis is key in helping patients to live longer and more productive, independent lives. This article presents the risk factors, signs, and symptoms of AD and reviews diagnostic and histological techniques and the most current treatments.
Key Words: Acetylcholine, acetylcholinesterase, Alzheimer’s disease, butyrylcholinesterase, choline acetyltransferase, N-methyl-d-aspartate, neuritic plaque, neurofibrillary tangle
Abbreviations used: ACh, acetylcholine; AChE, acetylcholinesterase; AD, Alzheimer’s disease; BChE, butyrylcholinesterase; ChAT, choline acetyltransferase; EEG, electroencephalography; FDA, Food and Drug Administration; NP, neuritic plaque; NFT, neurofibrillary tangle; NMDA, N-methyl-d-aspartate.
Alzheimer’s disease, a neurodegenerative disorder, was first described by Dr. Alois Alzheimer in a middle-aged woman with mental illness and a history of seizures. Her postmortem examination revealed abnormal clumps and tangled bundles of fibers in the brain. Today, these abnormal clumps and bundles of fibers, known as NPs and NFTs, respectively, are the pathologic hallmarks of AD.[1]
The social and economic impact of AD on society is tremendous. Since 1980 the number of Americans with AD has doubled to 4.5 million; by 2050, this number could exceed 13 million.[13] Worldwide, the current number of people affected is estimated to be 18 million, which will almost double to 34 million by 2025.[5] Unless medical advances are realized, these projections could lead to a global crisis. Furthermore, these numbers will only continue to increase as the population grows and life expectancy lengthens.
The cost of caring for an AD patient can be astronomical. The average life span after diagnosis is 6 to 9 years although some patients may survive for as many as two decades. As new therapies are discovered, the life expectancy for patients with AD will also increase. In the United States, the estimated cost for direct care of patients with AD is a staggering 110 billion dollars per year.[11]
The financial, emotional, and physical burdens for the caregiver of an AD patient are also considerable. Many productive members of society leave the work force to care for their ailing loved ones, assuming many of the tasks that patients can no longer perform. Ultimately, patients become completely dependent on the caregiver, resulting in economic and emotional difficulties for families and caregivers.
The Family Caregiver Alliance estimates that care-giving spouses between the ages of 66 and 96 years old who are experiencing mental or emotional strain have a 63% higher risk of dying than people the same age who are not caregivers.[12] According to the Alzheimer’s Association, the signs and symptoms of caregiver stress frequently include anger, anxiety, denial, exhaustion, health problems, guilt feelings, irritability, lack of concentration, sleeplessness, and social withdrawal. Hence, treating the caregiver becomes as important as treating the patient with AD.
Depression is common among AD caregivers. One study examined 42 couples equally distributed among early phase AD, ischemic stroke, and controls. Evaluation after 6 months showed that depression was significantly higher in AD and stroke caregivers compared to the controls. Furthermore, 21% of caregivers evidenced moderate to severe depression, and the percentage increased to 50% after 1 year. As the mental and physical health of caregivers declines, they may become unable to care for their loved one with AD.[1]
Risk Factors
Physicians must be aware of the differences between normal aging and the signs of dementia in middle-aged to elderly patients. There are many similarities between normal aging and dementia such as difficulties with memory and cognition, abnormal behavior, decreased ability to perform activities of daily living, brain atrophy, loss of neurons, and reduction of neurotransmitters. A dementing condition such as AD, however, is a progressive process that impairs social and professional functioning. Because AD is a degenerative brain disorder, it is assumed that the pathologic process begins years before its clinical manifestation.
Assessing risk factors and taking an extensive patient history are vital when interviewing patients with cognitive deficits. Age is the greatest risk factor because most AD patients are late middle-aged and older. About 3% of Americans between the ages of 65 and 74 years and almost half of individuals over the age of 85 years have the disease. In rare genetically linked cases associated with mutation of chromosomes 1, 14, and 21, the onset of symptoms could begin as early as the 30s or 40s.[4] In contrast, the ApoE4 allele is a known risk factor for late onset dementia (onset after age 65). A family history of AD increases the risk to 1.5 times if one parent has the disease and to 5 times if both parents are affected. Most patients with Down’s syndrome develop AD-like symptoms and pathology at 40 years and beyond. Mothers who give birth to children with Down’s syndrome have a higher than average risk of developing the disease.[4]
Other risk factors include traumatic brain injury, ethnicity, lower education level, gender, lifestyle, and environmental factors.[4] We and others have reported dementia in retired boxers who developed dementia and Parkinsonism and who had increased numbers of NPs and NFTs in certain brain regions at autopsy.[19]
African-Americans have four times as great a risk of developing AD as Caucasians. A study published in the Journal of the American Medical Association found that the risk for AD was almost twice as high for African-Americans compared to Africans from Nigeria.[15]
In a study conducted at the University of Pennsylvania, the onset of symptoms among Hispanic patients with AD was 6.8 years earlier than in Caucasians. According to a report from the Alzheimer’s Association, Hispanics may be at a greater risk for developing AD than any other ethnic group. It has been projected that the number of older Hispanics who suffer from AD or other dementias could increase from fewer than 200,000 today to as many as 1.3 million by 2050.[2,7] These data are of great interest because Hispanics are expected to become the largest minority group in the United States.
Lower education level is also a risk factor associated with AD. It may be that highly educated people develop more complex connections in the brains that compensate for the neuronal losses associated with AD.[3]
Although the reason is unclear, women have a greater risk of developing AD than men. It is unclear whether the risk reflects that women live longer than men and hence have an increased chance of developing the disease, or whether some other genetic or physical reason is involved. The results of studies on the role that estrogen and hormone replacement therapy plays in developing AD are mixed.[14]
Obesity in midlife, high total cholesterol level, nutrition, and hypertension are also considered by some as risk factors for developing dementia or AD later in life.[16]
Signs and Symptoms of AD
AD is a neurologic syndrome associated with insidious decline of cognitive and behavioral functions accompanied by changes in activities of daily living. Its neuropathologic correlates are increased density of NPs and NFTs in certain brain regions. Duration, onset, and evolution of clinical manifestations, together with associated, preceding, precipitating, and exacerbating factors and comorbid conditions, must be recorded and monitored carefully. Clinical deficits usually affect social and professional functioning, and focal neurologic signs are absent.[10]
The progression of AD can be classified by symptomatology into three stages. The first stage is mild or early stage, which is marked by symptoms that include misplacing objects, word-finding difficulty, visuospatial disorientation, short-term memory loss, changes in mood and behavior, personality alterations, loss of initiative, and denial of symptoms. Repeating and word-finding difficulties are common initial complaints.
Other interesting phenomena that we have reported are a preference for “sweets” and olfactory deficits in patients with mild to moderate degrees of dementia.[8,9,21,24] This same group of patients had large numbers of NPs and NFTs in the central connection of the olfactory system.[23] If unrecognized, this phenomenon could lead to nutritional problems that further complicate the course of the disease.
In the second stage, the moderate or midstage, the patient’s earlier symptoms worsen and begin to be accompanied by hallucinations, loss of language functions (circumlocution), wandering, violent behavior, and sleep disturbances. In this stage more supervision is required. Late in the midstage, patients exhibit short- and long-term memory loss, difficulty with familiar tasks, disorientation to time or place, impaired judgment, and problems with abstract thinking.
In the third stage, severe or later stage AD, patients become bedridden, stiff, unresponsive, incontinent, and unable to communicate. Most AD patients die of infections or pneumonia.
Caregivers should be aware of the symptoms that accompany the progression of AD. This information helps prepare caregivers for the challenges that they will confront and allows them the opportunity to seek appropriate advice from health care professionals. Throughout the patient’s clinical manifestation, side effects, in particular the extrapyramidal complications of neuroleptics and phenothiazines, and possible drug interactions need to be assessed closely.
Diagnostic Approach
A comprehensive assessment consisting of a thorough medical history with the help of a reliable caregiver, coupled with careful physical and neurologic examinations, is critical when diagnosing AD. Many clinical measures can be used to evaluate the neurobehavioral symptoms of AD patients. Options include the Mini-Mental State Examination, the Alzheimer’s Disease Assessment Scale, the Geriatric Depression Scale, and the Progressive Deterioration Scale for activities of daily living. Despite the available clinical tools, AD can only be diagnosed definitively by postmortem examination of the brain. Similarly, no reliable biomarkers are available.
All other causes of dementia must be ruled out through diagnostic tests such as a complete blood count; thyroid function studies; the venereal disease research laboratory test; serum levels of vitamin B12, folate, and homocystine; serum chemistries; the sedimentation rate; computed tomography; and magnetic resonance imaging. The last two tests are necessary to exclude structural, focal, or multifocal brain lesions. In atypical cases, positron emission tomography may be used to help distinguish AD from frontotemporal dementia. EEG is helpful in excluding seizure phenomena, which may occur in a small percentage of patients with AD or which may suggest a concomitant irritating brain lesion. When indicated clinically, the following tests may be performed: EEG, spinal fluid examination, tests for human immunodeficiency virus and other infectious agents, and immunologic studies.
The diagnosis of AD, based on the National Institute of Neurological Disorders and Stroke criteria, is categorized as probable, possible, and definite.[18] Probable AD refers to cases with a typical clinical course and negative confounding systemic or other neurologic conditions after patients have undergone complete medical and neurobehavioral examinations. Possible cases are considered to have consistent symptomatology accompanied by comorbid diseases that may complicate or exacerbate the patients’ symptoms. Definitive AD is reserved for clinically diagnosed patients with established autopsy findings.
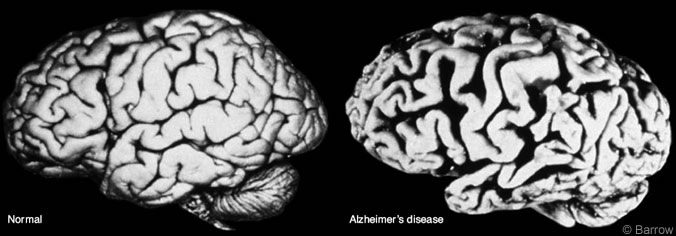
Neuropathology of AD
The structures in the brain most adversely affected by AD are the hippocampus, amygdala, entorhinal cortex, and basal forebrain nuclei. Many of these structures are components of the limbic system considered to be important in memory-related processes, emotional expression, and behavior. Such structures are supplied by cholinergic neurons that produce ACh, a putative neurotransmitter believed to be critical in maintaining higher cortical functions.[6] The activity of ChAT, the synthesizing enzyme for ACh, is also diminished significantly. Levels of other peptides (corticotropin releasing hormone and somatostatin) and neurotransmitters (norepinephrine) may be reduced. However, ACh is most studied in AD. For this reason cholinesterase inhibitors, which include tacrine, donepezil, rivastigmine, and galantamine, were the first line of drugs developed to treat AD.
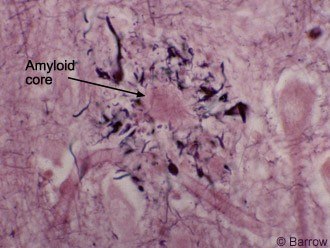
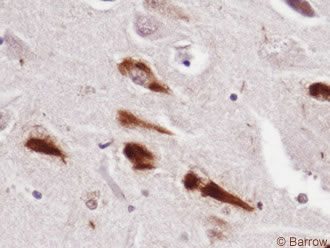
The neuropathology of AD may be grouped into gross and microscopic changes. Cerebral atrophy, volume loss, ventricular dilatation, and widened sulci are common and verified by computed tomography or magnetic resonance imaging (Fig. 1). Microscopically, high concentrations of NPs (Fig. 2) and NFTs (Fig. 3), the two histologic hallmarks of AD, are found in the hippocampus, entorhinal cortex, certain parts of the neocortex, and amygdala. Our studies have demonstrated large numbers of similar lesions in the central connections of the olfactory system.[20] Furthermore, the cholinergic neurons of the nucleus basalis of Meynert and synapses are lost.[6]
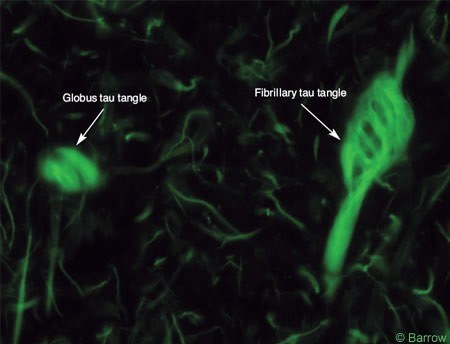
Recent investigations have shown that deposition of b-amyloid, a 40-42 amino acid molecule found in NPs, is cleaved from the amyloid precursor protein by b and g secretases. Separate studies have demonstrated that NFTs are composed of hyperphosphorylated tau, a microtubule-associated protein, important for maintaining normal cell structure, shape, and axoplasmic flow (Fig. 4).[17] These findings are extremely relevant because they have heightened worldwide interest in developing more definitive therapies.
Pharmacologic Treatment of AD
The treatment of AD must incorporate pharmacologic and nonpharmacologic strategies. It also must consider the healthcare needs of the caregivers.
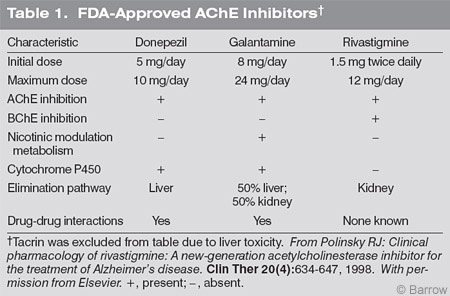
At present the FDA has approved five drugs for AD. The first four are central-acting cholinesterase inhibitors designed to ameliorate ACh deficiency by inhibiting AChE activity. The fifth is an NMDA antagonist (Table 1). Clinical trials using these agents have shown modest effects in AD, but none has been shown to arrest disease progression. Among the cholinesterase inhibitors, rivastigmine inhibits both AChE and BChE, both of which can hydrolyze ACh. Galantamine is believed to modulate nicotinic receptors. Donepezil is strictly an AChE inhibitor.
Such differences in pharmacologic action may be relevant in choosing appropriate medications. For instance, dual inhibition of AChE and BChE may be important because both enzymes can hydrolyze ACh, further reducing its level. Galantamine, through its nicotinic modulating effect, also may increase available ACh. Rivastigmine is the only drug excreted by the kidney and does not undergo hepatic metabolism, thus reducing the possibility of drug-to-drug or drug-disease interactions or both.
Potential adverse events associated with all cholinesterase inhibitors are nausea, vomiting, abdominal cramping, diarrhea, increased urination, and rhinorrhea. These drugs, however, should be used with caution in patients with recent myocardial infarction, unstable coronary artery disease, a history of gastrointestinal bleeding, and obstructive uropathy. When rivastigmine and galantamine are used, it is recommended that they be administered after complete meals to reduce the incidence of adverse events related to the gastrointestinal system.
Every naive patient must receive the lowest starting dose (5 mg daily for donepezil, 1.5 mg for rivastigmine twice daily usually after a complete breakfast and supper, and 8 mg daily for galantamine), which should be titrated at least every 4 weeks. The titration of each patient must be individualized. For instance, certain patients may respond best to 5 mg of donepezil and not to 10 mg, the maximum recommended dose. In other cases, rivastigmine may be increased from 1.5 mg twice daily to 1.5 mg after breakfast and 3 mg after supper before the dose is increased to 3 mg twice daily. It is equally important to remember that every patient does not have to be titrated to the maximum allowable dose. Higher doses may be reserved for patients who decline after a favorable initial response or who continue to deteriorate with a lower dosage.
Memantine, an NMDA-receptor antagonist, has been approved for moderately severe to severe dementia of the Alzheimer’s type. Although it is thought to have neuroprotective effects, memantine has been denied FDA approval for use with mild to moderate stages of the disease.
The introduction of different pharmacological agents has given physicians opportunities to switch patients from one cholinesterase inhibitor to another and to combine an NMDA-receptor antagonist with a cholinesterase inhibitor. When pursuing this approach, it is critical to consider the stage of the disease, cost of medication, and expected benefits.
Behavioral symptoms can pose a therapeutic challenge. Depression, when present, should be treated aggressively, preferably with selective serotonin reuptake inhibitors. The latter, however, can be associated with central nervous system symptoms as well as with gastrointestinal symptoms such as diarrhea caused by sertraline. Violent behavior, delusion, paranoia, and hallucination may require atypical neuroleptics, which could give rise to excessive sedation and extrapyramidal or Parkinsonian symptoms. The latter signs must be identified to distinguish them from patients with AD with Lewy bodies.[22,25] When abnormal behaviors occur, their onset and precipitating and exacerbating factors must be carefully noted to maximize the effects of medications and behavioral techniques (see Nonpharmacological Interventions for Use in Dementia in this issue).
Most patients with AD suffer from other conditions that require daily medications. It is imperative that clinical deterioration be assessed prudently to exclude the role of comorbid conditions, drug interactions, and disease progression.
Nonpharmacologic approaches consist of dietary supplements, mental and physical exercises, and the use of behavioral techniques. For instance, foods high in antioxidants such as blueberries, strawberries, purple grape juice, and spinach have neuroprotective properties that improve the health of the brain. In animal experiments, a low-calorie diet has been associated with a longer life span and improved learning whereas low serum cholesterol and previous exposure to cholesterol-lowering agents may reduce the risk for AD. Other neuroprotective agents such as nerve growth factor, vitamin E, and nonsteroidal anti-inflammatory drugs have also been found to reduce the risk of developing AD. Mental and physical exercises are essential to maintain a normal sense of well being.
Understanding the clinical course and symptomatology of AD is important to both physicians and caregivers. Instructions must be simplified, and triggers for emotional outbursts or abnormal behavior should be avoided. As the disease progresses, the use of psychopharmacologic agents such as atypical neuroleptics and anxiolytics becomes inevitable. When the time comes, low doses must be prescribed and titration must proceed slowly.
Conclusion
AD is a diagnosis by exclusion. The etiology remains elusive, but risk factors have been identified. There is no cure, but symptoms are treatable. It is hoped that increased clinical and basic science research will continue to unravel the complex mechanisms that underlie the pathogenesis of AD and lead to more definitive and safer therapy.
References
- ADEAR. Alzheimer’s Disease Education and Referral Center: What is Alzheimer’s Disease (AD)? Available at: https://www.alzheimers.org. [Accessed 2005]
- Alzheimer’s Association: Hispanics/Latinos and Alzheimer’s disease. Report from the Alzheimer’s Association. Available at: https://www.alz.org/national/documents/report_hispanic.pdf. [Accessed May 2004]
- Alzheimer’s Society Dementia Care and Research: Alzheimer’s Society Information Sheet. Available at: https://www.alzheimers.org.uk/Facts_about_dementia/Risk_factors/info_amIatrisk.htm. [Accessed October 2001]
- American Federation for Aging Research: American Federation for Aging Research, Available at: https://www.Infoaging.org. [Accessed September 11, 2003]
- American Health Assistance Foundation (AHAF): About Alzheimer’s disease information page. Available at: https://www.ahaf.org/alzdis/about/adabout.htm. [Accessed 2005]
- Bakry NM, Reyes PF, Golden GT: Regional distribution of cholinergic sites in Alzheimer’s disease (abstract). 46th Annual Meeting of the American Academy of Neurology. Taken from: Neurology 44 (Suppl 2):A389, (Abstract 1023P), 1994.
- Clark CM, DeCarli C, Mungas D, et al: Earlier onset of Alzheimer disease symptoms in Latino individuals compared with Anglo individuals. Arch Neurol 62:774-778, 2005
- Doty RL, Perl DP, Steele JC, et al: Olfactory dysfunction in three neurodegenerative diseases. Geriatrics 46 Suppl 1:47-51, 1991
- Doty RL, Reyes PF: Olfactory dysfunction in Alzheimer’s disease. Ann NY Acad Sci 510:260-262, 1988
- Doty RL, Reyes PF, Gregor T: Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res Bull 18:597-600, 1987
- Ernst RL, Hay JW: The US economic and social costs of Alzheimer’s disease revisited. Am J Public Health 84:1261-1264, 1994
- Healthlink MCoW: With Alzheimer’s, the caregiver is a patient too. Available at: https://www.healthlink.mcw.edu/article/1031002313.html [Accessed 2003]
- Hebert LE, Scherr PA, Bienias JL, et al: Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Arch Neurol 60:1119-1122, 2003
- Henderson VW: Estrogen-containing hormone therapy and Alzheimer’s disease risk: Understanding discrepant inferences from observational and experimental research. Neuroscience, 2005 (Epub ahead of print).
- Hendrie HC, Ogunniyi A, Hall KS, et al: Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA 285:739-747, 2001
- Kivipelto M, Ngandu T, Fratiglioni L, et al: Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62:1556-1560, 2005
- Klunk WE, Abraham DJ: Filamentous proteins in Alzheimer’s disease: New insights through molecular biology. Psychiatr Dev 6:121-152, 1988
- McKhann G, Drachman D, Folstein M, et al: Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939-944, 1984
- Reyes PF, Booth K, Barbaria A, et al: Dementia among retired elderly boxers (abstract). Presented at the 51st Annual Meeting of the American Academy of Neurology, Chicago, IL, April 1989
- Reyes PF, Deems DA, Suarez MG: Olfactory-related changes in Alzheimer’s disease: A quantitative neuropathologic study. Brain Res Bull 32:1-5, 1993
