Department of Neurology
Our Mission
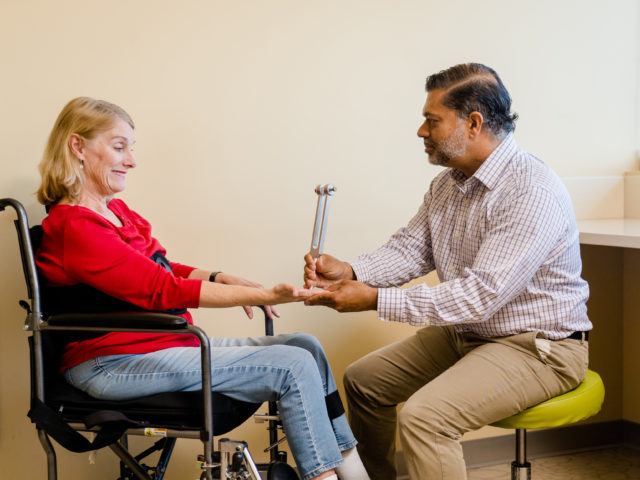
The Department of Neurology at Barrow Neurological Institute, chaired by Brad A. Racette, MD, FAAN, is an academic neurology department with a traditional tripartite mission: providing cutting-edge clinical care to patients with neurologic diseases, training the next generation of physician-leaders, and conducting clinically relevant research. The Barrow Department of Neurology has a strong commitment to global neurology across these three mission areas.
The Department of Neurology is composed of a diverse faculty of more than 60 neurologists, scientists, and other providers who deliver compassionate and patient- and caregiver-focused care across 13 subspecialties. Barrow neurologists are internationally recognized leaders in their respective specialties. Our physicians are deeply committed to health equity, as evidenced by unique outreach programs in most subspecialties, a free neurology clinic at St. Vincent DePaul, and a continuity neurology clinic on the San Carlos Apache Reservation.
Barrow Neurology trains residents and fellows to be national and international leaders in clinical care, service, global neurology, and research. Trainees benefit from a rich clinical environment and talented clinician-educators who oversee our training program. Student-doctors from Creighton University Medical School and the University of Arizona College of Medicine learn neurology from the Barrow residents and attendings in an institute dedicated to clinical neuroscience. Trainees can also experience global neurology by rotating at Chris Hani Baragwanath Hospital in Soweto, South Africa through our Barrow Global Neurology Program, offered in partnership with the University of the Witwatersrand in Johannesburg.
Barrow researchers are dedicated to clinically relevant research that is tightly aligned with the needs of our patients. Barrow Department of Neurology research spans the spectrum from translational neuroscience to clinical research, including neuroepidemiology data science, neuroimaging, biofluid multi-omic analyses, basic science, and investigator-initiated clinical trials. The Barrow Neuro Analytics Center is a 10,099-square-foot dry lab research facility focusing on data science from large populations to deep phenotyping of human research participants and cells.
Designated Centers of Excellence and Recognitions
- ALS – ALS Association Certified Treatment Center of Excellence
- Alzheimer’s & Cognitive Disorders – Lewy Body Dementia Association Center of Excellence
- Ataxia – National Ataxia Foundation Center of Excellence
- Epilepsy – National Association of Epilepsy Centers – Level 4 Comprehensive Epilepsy Center
- Hereditary Neuropathy – Hereditary Neuropathy Foundation CMT Center of Excellence
- Huntington’s Disease – Huntington’s Disease Society of America Center of Excellence
- Multiple Sclerosis – National Multiple Sclerosis Society Center for Comprehensive Care
- Muscular Dystrophy – Muscular Dystrophy Association Care Center
- Parkinson’s Disease – Parkinson Foundation Center of Excellence
- Stroke – The Joint Commission and American Heart Association/American Stroke Association Certified Comprehensive Stroke Center; American Heart Association 2024 Get With The Guidelines Stroke Gold Plus Quality Achievement Awards, Target: Stroke Honor Roll, Target: Type 2 Diabetes Honor Roll
- Alzheimer’s and Memory Disorders
- Balance Disorders & Neuro-Otology
- Brain Injury & Sports Neurology
- Epilepsy
- Headache Medicine
- Movement Disorders
- Multiple Sclerosis & Neuro-Immunology
- Neurocritical Care
- Neuro-Endocrinology & Pituitary
- Neuromuscular Disorders
- Neuro-Ophthalmology
- Neurovascular & Stroke
Specialists
Faculty

Gill Nelson, PhD, MSc
Neurology
Fellows
Residents
Medical Education
Clinical and Laboratory Research
Enroll HD is a longitudinal, observational, multinational study that will integrate two existing Huntington’s Disease registries, REGISTRY in Europe and COHORT in North America and Australia, while also expanding to include sites in Latin America and Asia. The goal is the build a large and...
The purpose of this program is to create a postmortem (after death) tissue bank of spinal cord, muscle, and brain tissue samples from both amyotrophic lateral sclerosis (ALS) patients and those without the disease (control subjects). Tissue donation provides an important and vital resource toward...
The purpose of this study is to determine whether magnetic resonance imaging (MRI) cytography (a type of noninvasive body scan) is useful in establishing disease severity in individuals with amyotrophic lateral sclerosis (ALS). MRI cytography will be compared to measures typically taken at clinic visits...
The Lysosomal Storage Disorders (LSD) Registry Program is a multicenter, international, observational program for people with certain rare diseases. It is designed to track the natural history and outcomes of patients. Currently, patients diagnosed with Pompe disease may participate in this registry program. No experimental...
About FDA-approved multiple sclerosis (MS) disease-modifying therapies (DMTs) target the relapsing phase of MS but have minimal impact once the progressive phase has begun. It is unclear if, in the relapsing phase, there is an advantage of early aggressive therapy with respect to preventing long-term...
The overall goal of ADNI3 is to determine the relationships among the clinical, cognitive, imaging, genetic and biochemical biomarker characteristics of the entire spectrum of Alzheimer’s disease (AD), as the pathology evolves from normal aging through very mild symptoms, to mild cognitive impairment (MCI), to...
A Phase 3 study to evaluate efficacy, safety, and tolerability of ampreloxetine (TD-9855) in subjects with primary autonomic failures (MSA, PD, or PAF) and snOH with up to 4 weeks of treatment. A Phase 3, randomized, double-blind, placebo-controlled, parallel-group, multicenter study to evaluate efficacy, safety,...
This is an observational, multicenter, longitudinal cohort study to characterize adults with Down syndrome ages 25 years and above enrolled at specialized care centers. The aim is to assess changes in cognition, behavior, function, and health over approximately 24 months. Blood will be collected for...
The purpose of this study is to identify biomarkers to serve as quantifiable measures of Edaravone effects in ALS in those who are initiating clinically prescribed Edaravone treatment for the first time or within one month of consenting. Participants in the study will be followed...
The purpose of this study is to assess the clinical efficacy, safety and tolerability of Rozanolixizumab, which is a type of antibody that helps breakdown harmful auto-antibodies in generalized myasthenia gravis patients. Rozanolixizumab will be administered through a 30-minute subcutaneous infusion (i.e. a small needle...
The purpose of this study is to evaluate the efficacy, safety, and tolerability of HYQVIA/HyQvia as a maintenance therapy to prevent relapse and for the treatment of CIDP. Subjects must be on a stable dosing regimen of IVIG therapy for at least 12 weeks prior...
Blinded comparison of different alpha-synuclein seeding assays as cutaneous biomarkers of Lewy body dementias. Beach-NIH/R01 (NS118669-01). Purpose The purpose of this research is to determine the ability of skin biopsy analysis to predict a diagnosis of Lewy body dementia, and to determine whether the results...
PPMI 2.0 is a broad program, expanding the goals of the original PPMI study (NCT01141023), that includes this PPMI 2.0 Clinical protocol, as well as the PPMI 2.0 Remote, PPMI 2.0 Digital Applications and PPMI 2.0 Online protocols. All participants in PPMI 2.0 will be...
The purpose of this platform trial is to evaluate the efficacy of multiple study drugs at once, as compared with placebo, on ALS disease progression. New treatment regimens (drugs) will be added as they become available within the trial, meaning that multiple drug treatment regimens...
Full Study Name: A Dose Selection Trial of Light Therapy for Impaired Sleep in Parkinson’s Disease (ENLITE PD – NN110) Description: The ENLITE PD – NN110 study is a 16-week, phase II, multi-center, randomized, parallel-group, dose-selection trial of light therapy in participants with Parkinson’s Disease...
Alzheimer’s disease (AD) is a fatal neurodegenerative disease that is manifested by progressive cognitive deficits including memory loss followed by loss of independent function as well as neuropsychiatric symptoms such as apathy, depression, anxiety, agitation and psychosis. JNJ-63733657 is a humanized monoclonal anti-tau antibody which...
This is a phase 3 double blind, placebo controlled study evaluating the efficacy and safety of AL001 administered intravenously in participants at risk for or with frontotemporal dementia due to heterozygous mutations in the progranulin gene. Full Name: A Phase 3 Study to Evaluate Efficacy...
The primary objective is to evaluate the long-term safety and tolerability of aducanumab after a wash-out period imposed by discontinuation of feeder studies in participants who had previously received aducanumab (i.e. previously treated participants) or who had previously received placebo (i.e. treatment-naïve participants). Full Name:...
A Phase 3, Multi-center, Randomized, Double-blind, Parallel-group, Placebo-controlled Clinical Trial to Evaluate the Efficacy and Safety of Sodium Oligomannate (GV-971) in Treatment of Mild to Moderate Alzheimer’s Disease (GREEN MEMORY: GREen Valley 971 EvaluatioN Memory) The purpose of this study is to evaluate the safety,...
This research is being done to figure out whether treatment for sleep apnea, in people who have had a stroke or TIA, improves recovery from stroke, and helps prevent future stroke, heart problems, and death. The intervention being tested is called continuous positive airway pressure...
StATins Use in intRacereberal hemorrhage patieNts (SATURN) This research is being done to find out if it is better to continue or discontinue statin drugs in people who had a brain hemorrhage while taking a statin drug. Statin drugs help prevent heart disease and ischemic...
Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multicenter, Phase 3 Study to Evaluate the Efficacy and Safety of Intravenous BIIB093 (Glibenclamide) for Severe Cerebral Edema following Large Hemispheric Infarction This is a Phase 3 study, which means that BIIB093 (study drug) has already been investigated in previous clinical research studies...
Phase 2A Safety, Tolerability, Pharmacokinetic, and Pharmacodynamic Study of Intravenous ANX005 in Subjects with Amyotrophic Lateral Sclerosis The purpose of this study is to evaluate the safety and tolerability of ANX005, which is a monoclonal antibody that is being developed to treat ALS in adult...
Efficacy and Safety Study of Oral Edaravone Administered to Subjects with ALS The purpose of this trial is to evaluate and compare the efficacy, safety, tolerability, and changes in nerve conduction tests of 2 different dosing regimens of oral edaravone (study drug) in participants with...
A Phase 3, Multi-Center, Double-blind, Randomized, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of Reldesemtiv in Patients with ALS The purpose of this trial is to evaluate the effect of reldesemtiv (study drug) v. placebo on ALSFRS-R, FVC, quality of life scales, muscle and...
A Phase 2a Open-Label, Multi-Center Study to Evaluate the Safety and Tolerability of Multiple Doses of AT-1501 in Adults with ALS The purpose of this trial is to determine the safety and tolerability of multiple doses of AT-1501 (study drug) in participants with ALS. Additionally,...
Long-Term, Observational, Registry of Patients with Generalized Myasthenia Gravis Who Have Received Treatment With Complement C5 Inhibition Therapies This is a registry study that focuses on patients with Myasthenia Gravis (MG) who have used complement inhibitors. Soliris is an example of this type of medication....
A Multi-Center, Longitudinal Study of the Natural History of Subjects with Limb Girdle Muscular Dystrophy (LGMD) Type 2E (LGMD2E/R4), Type 2D (LGMD2D/R3), and Type 2C (LGMD2C/R5) The purpose of this study is to learn about the natural progression of LGMD sarcoglycanopathies (types 2E, 2D, and...
Reliability Studies of Multipoint Incremental MUNE in the Lower Extremities The purpose of this study is to evaluate the application of multipoint incremental motor unit number estimate (MIMUNE), an electromyography (EMG) technique, in 2 distinct muscles of the leg called the extensor digitorum brevis and...
Electrical Impedance Myography Technology for Quantitative, At-home Muscle Assessment in Amyotrophic Lateral Sclerosis (EIM-At-Home) This research is being performed to evaluate the reliability and ease of use of the improved mScan™ EIM (electrical impedance myography) device as a potential biomarker for ALS for patients to...
Lysosomal Storage Disorders (LSD) Registry Program – Pompe Registry The LSD Registry program is a multi-center, international, observational program for patients with certain rare diseases designed to track the natural history and outcomes of patients. Currently, patients diagnosed with Pompe disease may participate in this...
Phase 1/2, Dose-escalation Study to Evaluate the Safety, Tolerability and Efficacy of a Single Intravenous Infusion of SPK-3006 in Adults with Late-onset Pompe Disease The purpose of this study is to evaluate the safety, tolerability, and efficacy of SPK-3006, which is a novel investigational gene...
Target ALS Biomarker Study: Longitudinal Biofluids, Clinical Measures, and At-Home Measures The purpose of this study is to generate a biorepository of longitudinal blood (plasma and serum), cerebral spinal fluid (CSF), and urine linked to genetics and clinical information that are then made available to...
What is the purpose of the study? The purpose of this study is to see whether at-home measurements are dependable and can help with tracking disease progression. What procedures will I participate in if I choose to enroll in the study? You will be required...
What is the FASTEST Trial? Barrow Neurological Institute is conducting a research study of the emergency treatment of patients with bleeding in the brain, also called intracerebral hemorrhage. Barrow Neurological Institute has joined a partnership of over 100 other hospitals and mobile stroke units across North...
DISCOVERY is a prospective, multicenter, observational, nested-cohort study with the overarching objectives to identify specific stroke subtypes associated with risk of PSCID in diverse US populations and elucidate mechanisms of PSCID through systematic assessment of the effect of acute stroke and its interaction with antecedent...
MultiStem is an allogeneic, regenerative medicine advanced therapy indicated for treatment of acute ischemic stroke within 36 hours after symptom onset. The primary objective of this study is to evaluate the efficacy of MultiStem on functional outcome in subjects with ischemic stroke. This is a...
This is a randomized clinical trial to determine the efficacy and safety of andexanet compared to usual care in patients presenting with acute intracerebral hemorrhage within 6 hours of symptom onset (from the baseline scan) and within 15 hours of taking an oral FXa inhibitor...
This is a 12-week, double-blind, placebo-controlled, randomized, parallel-group, multicenter study of the safety and efficacy of JZP385 in the treatment of adult participants with moderate to severe essential tremor. Purpose The purpose of this study is to find out whether an investigational drug called JZP385...
Purpose This study will test a new deep brain stimulation (DBS) site that is expected to better address these features of Parkinson’s disease. Who Can Participate Parkinson’s disease patients that are discussing deep brain stimulation therapy with their physician and have difficulties with gait, balance,...
The purpose of this trial is to develop a new magnetic resonance imaging (MRI) method to accurately diagnose, predict, track progression, and monitor the response to treatment in Parkinson’s disease. Purpose This study is being done in hopes of developing new MRI methods to accurately...
Development of a central repository for Parkinson’s disease-related genomic data for future research. Purpose This study will obtain genetic samples to investigate the genetic link to Parkinson’s disease. Patients can receive their genetic results if they wish. Who Can Participate Male and female patients over...
A phase-2B, double-blind, randomized controlled trial to evaluate the activity and safety of inebilizumab in anti-NMDA receptor encephalitis and assess markers of disease. About There are currently no medicinal products approved for the treatment of anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis, a rare disease. NMDAR encephalitis is...
A pilot study examining the effects of pre-treatment with Korlym before performing bilateral inferior petrosal sinus sampling in patients with Cushing syndrome. Purpose This study will assess if by increasing ACTH and cortisol levels by virtue of antagonizing the glucocorticoid receptors with Korlym which will...
A phase 3, double-blind, placebo-controlled, randomized-withdrawal study of the efficacy and safety of relacorilant. Purpose This study will evaluate the efficacy and safety of GR antagonism by relacorilant, with an emphasis on two robust and validated endpoints, blood glucose control and blood pressure (BP) control,...
A multicenter, randomized, parallel-arm, placebo-controlled (double-blind) and active controlled (open-label) trial to compare the efficacy and safety of once-weekly lonapegsomatropin with placebo and a daily somatropin product in adults with growth hormone deficiency. Purpose Primary objective is to evaluate the efficacy of once-weekly lonapegsomatropin compared...
A multicenter, open-label, extension trial to investigate long term efficacy and safety of lonapegsomatropin in adults with growth hormone deficiency. Purpose This is a phase 3 open-label multicenter extension trial designed to evaluate the long-term safety and efficacy of lonapegsomatropin administered once-weekly. The trial participants...
Purpose The purpose of this study is to evaluate the safety and efficacy of botox for the treatment of upper limb essential tremor. Who can participate? Patients with diagnosed essential tremor >3 years Essential tremor requirements (all in dominant limb): Principal Investigator
Purpose The purpose of the study is to assess the feasibility and impact of time-restricted eating on cognitive performance and blood biomarkers of metabolic health in adults with mild cognitive impairment. We will collect characteristics that are associated with cognitive function and cognitive decline (e.g....
Purpose The purpose of this study is to assess the efficacy of BIA 28-6156 in delaying meaningful clinical motor progression in subjects with Parkinson’s disease (PD) who have a pathogenic variant in the GBA1 gene (GBA-PD). Who can participate? Male and female subjects between 35...
Purpose The ABATE Study is testing an investigational vaccine for Alzheimer’s disease in people with Down Syndrome. We want to see if the vaccine is safe. We also want to see if it slows the progression of Alzheimer’s disease in people with Down syndrome. The...
Purpose This study is a multicenter, biologic samples, and health-related data collection. The purpose of this study is to establish a collection of health-related data and biological samples that will enable researchers to understand the cause of cryptogenic new-onset refractory status epilepticus (NORSE), to identify...
Purpose This study is a randomized, sham-controlled, double-blinded, multi-center study, followed by optional open-label extension (OLE). The purpose of this study is to evaluate efficacy, safety, and tolerability of repeated, daily sessions of transcranial cathodal direct current stimulation (tDCS). Who can participate Adults 18 years...
Purpose This study is a prospective observational cohort study of the association between early seizures (ES) and periodic discharges (PD) after acute brain injury (ABI) and the subsequent development of epilepsy. The purpose of this study is to assess the relationship between early seizures and...
Purpose This is an interventional, prospective, multicenter, open-label, post-market release clinical study with a 3-year follow-up period. The purpose of this post-approval study is to further evaluate the long-term safety and effectiveness of Medtronic DBS therapy for epilepsy on seizure reduction in newly implanted patients...
Purpose Epilepsy Learning Health System (ELHS), Epilepsy Surgical Materials Feedback Survey, and Mindset The purpose of the network is to improve seizure control and quality of life for people with epilepsy through a learning healthcare system, in which clinical data is collected, analyzed, and rapidly...
About the Study This study, funded by the State of Arizona Governor’s Office through the American Rescue Plan Act, aims to analyze if long COVID: Eligibility Patients are eligible to participate in the study if they are: Principal Investigators Enrollment Contact Phone: (602) 406-4280Email: MovementResearch@DignityHealth.org
Purpose The purpose of this study is to test two different doses of tominersen versus placebo to select a dose that could potentially benefit individuals with prodromal (subtle, nonspecific signs of Huntington’s disease (HD)) or early stage manifest HD (definite signs of a motor disorder...
About The long-term health outcomes of people living with multiple sclerosis (MS) and other neuroImmunology diseases are not well known. Barrow Neurological Institute is building a database that stores patient health data used to gain knowledge and identify trends in outcomes in patients suffering from...
About MS causes significant disability and costs in those affected, but those burdens are not distributed equally. Minority populations including Black and Latinx have worse outcomes in MS than whites. The reasons for these disparities are not well understood but are likely in part due...
About Multiple sclerosis is a chronic, debilitating disease of the central nervous system characterized byneuroinflammation, focal demyelination, gliosis, axonal degeneration, and neuronal loss. Asremyelination is both highly variable and associated with improvement of symptoms, therapies thatfoster remyelination represent an opportunity for repair prior to irreversible...
About Knowledge regarding MS pathogenesis is largely derived from animal models or studies on peripheral blood or cerebrospinal fluid (CSF) from patients. Cellular analyses of these samples have been limited to low resolution flow cytometric assessments of immune cell populations, and have been unable to...
About The development of disease modifying therapies for relapsing forms of multiple sclerosis (MS) has led to significant decrease of morbidity for people with MS. For individuals with relapsing-remitting MS, disability worsening starts from disease onset, and more than 50% of patients evolve to secondary...
A Randomized, Double-blind, Placebo-Controlled, Multicenter Phase 3 Study to Evaluate the Safety, Tolerability, and Efficacy of XEN1101 as Adjunctive Therapy in Focal-Onset Seizures (Xenon) Purpose This is a Phase 3, multicenter, randomized, double-blind, placebo-controlled study to evaluatethe clinical efficacy, safety, and tolerability of XEN1101 administered...
A Phase 2/3 Multicenter, Randomized, Double-Blind, Placebo-Controlled, Study to Evaluate the Efficacy, Safety and Tolerability of BHV-7000 in Subjects with Refractory Focal Onset Epilepsy (RISE2) Purpose This is a global, multicenter, randomized, double-blind, placebo-controlled 3-arm study designed to assess the efficacy and safety of BHV-7000...
A Phase II Clinical Trial for the Assessment of Biomarker Trajectory in Patients withMild Cognitive Impairment Due to Alzheimer’s Disease Treated with Lenalidomide About the Study This study, funded by Arizona Alzheimer’s Consortium aims to repurpose the anti-cancer drug lenalidomide and evaluate the safety of...
Research Laboratories